Recognition of phylogenetically diverse pathogens through enzymatically amplified recruitment of RNF213
- PMID: 39375464
- PMCID: PMC11549300
- DOI: 10.1038/s44319-024-00280-w
Recognition of phylogenetically diverse pathogens through enzymatically amplified recruitment of RNF213
Abstract
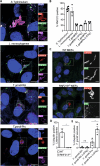
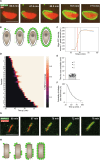
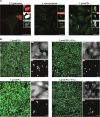
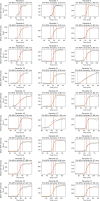
Innate immunity senses microbial ligands known as pathogen-associated molecular patterns (PAMPs). Except for nucleic acids, PAMPs are exceedingly taxa-specific, thus enabling pattern recognition receptors to detect cognate pathogens while ignoring others. How the E3 ubiquitin ligase RNF213 can respond to phylogenetically distant pathogens, including Gram-negative Salmonella, Gram-positive Listeria, and eukaryotic Toxoplasma, remains unknown. Here we report that the evolutionary history of RNF213 is indicative of repeated adaptation to diverse pathogen target structures, especially in and around its newly identified CBM20 carbohydrate-binding domain, which we have resolved by cryo-EM. We find that RNF213 forms coats on phylogenetically distant pathogens. ATP hydrolysis by RNF213's dynein-like domain is essential for coat formation on all three pathogens studied as is RZ finger-mediated E3 ligase activity for bacteria. Coat formation is not diffusion-limited but instead relies on rate-limiting initiation events and subsequent cooperative incorporation of further RNF213 molecules. We conclude that RNF213 responds to evolutionarily distant pathogens through enzymatically amplified cooperative recruitment.
Keywords: Host-pathogen Interaction; Innate Immunity; Pattern Recognition Receptor; Positive Selection; Ubiquitylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ahel J, Fletcher A, Grabarczyk DB, Roitinger E, Deszcz L, Lehner A, Virdee S, Clausen T (2021) E3 ubiquitin ligase RNF213 employs a non-canonical zinc finger active site and is allosterically regulated by ATP. Preprint at 10.1101/2021.05.10.443411
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

