TRPC5 controls the adrenaline-mediated counter regulation of hypoglycemia
- PMID: 39375537
- PMCID: PMC11612138
- DOI: 10.1038/s44318-024-00231-0
TRPC5 controls the adrenaline-mediated counter regulation of hypoglycemia
Abstract
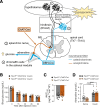
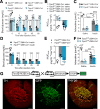
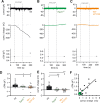
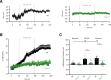
Hypoglycemia triggers autonomic and endocrine counter-regulatory responses to restore glucose homeostasis, a response that is impaired in patients with diabetes and its long-term complication hypoglycemia-associated autonomic failure (HAAF). We show that insulin-evoked hypoglycemia is severely aggravated in mice lacking the cation channel proteins TRPC1, TRPC4, TRPC5, and TRPC6, which cannot be explained by alterations in glucagon or glucocorticoid action. By using various TRPC compound knockout mouse lines, we pinpointed the failure in sympathetic counter-regulation to the lack of the TRPC5 channel subtype in adrenal chromaffin cells, which prevents proper adrenaline rise in blood plasma. Using electrophysiological analyses, we delineate a previously unknown signaling pathway in which stimulation of PAC1 or muscarinic receptors activates TRPC5 channels in a phospholipase-C-dependent manner to induce sustained adrenaline secretion as a crucial step in the sympathetic counter response to insulin-induced hypoglycemia. By comparing metabolites in the plasma, we identified reduced taurine levels after hypoglycemia induction as a commonality in TRPC5-deficient mice and HAAF patients.
Keywords: Adrenaline Secretion; Calcium Signaling; Chromaffin Cells; Hypoglycemia Associated Autonomic Failure; TRPC5 Channels.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures





References
-
- Adachi A, Kobashi M, Funahashi M (1995) Glucose-responsive neurons in the brainstem. Obes Res 3(Suppl 5):735S–740S - PubMed
-
- Battezzati A, Benedini S, Fattorini A, Piceni Sereni L, Luzi L (2000) Effect of hypoglycemia on amino acid and protein metabolism in healthy humans. Diabetes 49:1543–1551 - PubMed
-
- Bauer CC, Minard A, Pickles IB, Simmons KJ, Chuntharpursat-Bon E, Burnham MP, Kapur N, Beech DJ, Muench SP, Wright MH et al (2020) Xanthine-based photoaffinity probes allow assessment of ligand engagement by TRPC5 channels. RSC Chem Biol 1:436–448
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

