Proapoptotic activity of JNK-sensitive BH3-only proteins underpins ovarian cancer response to replication checkpoint inhibitors
- PMID: 39375715
- PMCID: PMC11457406
- DOI: 10.1186/s12943-024-02125-5
Proapoptotic activity of JNK-sensitive BH3-only proteins underpins ovarian cancer response to replication checkpoint inhibitors
Abstract
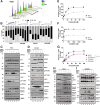
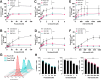
Recent studies indicate that replication checkpoint modulators (RCMs) such as inhibitors of CHK1, ATR, and WEE1 have promising monotherapy activity in solid tumors, including platinum-resistant high grade serous ovarian cancer (HGSOC). However, clinical response rates are generally below 30%. While RCM-induced DNA damage has been extensively examined in preclinical and clinical studies, the link between replication checkpoint interruption and tumor shrinkage remains incompletely understood. Here we utilized HGSOC cell lines and patient-derived xenografts (PDXs) to study events leading from RCM treatment to ovarian cancer cell death. These studies show that RCMs increase CDC25A levels and CDK2 signaling in vitro, leading to dysregulated cell cycle progression and increased replication stress in HGSOC cell lines independent of homologous recombination status. These events lead to sequential activation of JNK and multiple BH3-only proteins, including BCL2L11/BIM, BBC3/PUMA and the BMF, all of which are required to fully initiate RCM-induced apoptosis. Activation of the same signaling pathway occurs in HGSOC PDXs that are resistant to poly(ADP-ribose) polymerase inhibitors but respond to RCMs ex vivo with a decrease in cell number in 3-dimensional culture and in vivo with xenograft shrinkage or a significantly diminished growth rate. These findings identify key cell death-initiating events that link replication checkpoint inhibition to antitumor response in ovarian cancer.
Keywords: ATR inhibitor; Apoptosis; BH3 proteins; CHK1 inhibitor; High grade serous ovarian cancer; Replication stress; WEE1 inhibitor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

