Senescent cells promote breast cancer cells motility by secreting GM-CSF and bFGF that activate the JNK signaling pathway
- PMID: 39375718
- PMCID: PMC11457416
- DOI: 10.1186/s12964-024-01861-x
Senescent cells promote breast cancer cells motility by secreting GM-CSF and bFGF that activate the JNK signaling pathway
Abstract
Background: Cellular senescence can be induced in mammalian tissues by multiple stimuli, including aging, oncogene activation and loss of tumor suppressor genes, and various types of stresses. While senescence is a tumor suppressing mechanism when induced within premalignant or malignant tumor cells, senescent cells can promote cancer development through increased secretion of growth factors, cytokines, chemokines, extracellular matrix, and degradative enzymes, collectively known as senescence-associated secretory phenotype (SASP). Previous studies indicated that senescent cells, through SASP factors, stimulate tumor cell invasion that is a critical step in cancer cell metastasis.
Methods: In the current study, we investigated the effect of senescent cells on the motility of breast cancer cells, which is another key step in cancer cell metastasis. We analyzed the motility of breast cancer cells co-cultured with senescent cells in vitro and metastasis of the breast cancer cells co-injected with senescent cells in orthotopic xenograft models. We also delineated the signaling pathway mediating the effect of senescent cells on cancer cell motility.
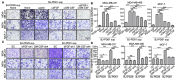
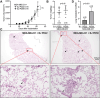
Results: Our results indicate that senescent cells stimulated the migration of breast cancer cells through secretion of GM-CSF and bFGF, which in turn induced activation of the JNK pathway in cancer cells. More importantly, senescent cells promoted breast cancer metastasis, with a minimum effect on the primary tumor growth, in orthotopic xenograft mouse models.
Conclusions: These results have revealed an additional mechanism by which senescent cells promote tumor cell metastasis and tumor progression, and will potentially lead to identification of novel targets for cancer therapies that suppress metastasis, the major cause of cancer mortality.
Keywords: Breast cancer; GM-CSF; JNK; Metastasis; Migration; Senescence; bFGF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Campisi J. Cancer, aging and cellular senescence. vivo. 2000;14(1):183–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

