Clinical characterization, prognostic, and predictive values of HER2-low in patients with early breast cancer in the PALLAS trial (ABCSG-42/AFT-05/BIG-14-13/PrE0109)
- PMID: 39375745
- PMCID: PMC11459983
- DOI: 10.1186/s13058-024-01899-2
Clinical characterization, prognostic, and predictive values of HER2-low in patients with early breast cancer in the PALLAS trial (ABCSG-42/AFT-05/BIG-14-13/PrE0109)
Abstract
Background: Bidirectional crosstalk between HER2 and estrogen receptor (ER) pathways may influence outcomes and the efficacy of endocrine therapy (ET). Low HER2 expression levels (HER2-low) have emerged as a predictive biomarker in patients with breast cancer (BC).
Methods: PALLAS is an open, international, phase 3 study evaluating the addition of palbociclib for 2 years to adjuvant ET in patients with stage II-III ER-positive/HER2-negative BC. To assess the impact of HER2 expression on patient outcomes in the phase III PALLAS trial, we analyzed (1) the association between rate of HER2-low with demographic and clinicopathological parameters, (2) the prognostic value of HER2-low status on invasive disease-free survival (iDFS), distant relapse-free survival (DRFS), and overall survival (OS) and (3) HER2 expression's value as a predictive biomarker of response to palbociclib. HER2-low was defined as HER2 immunohistochemistry (IHC) 1 + or IHC 2 + with negative in situ hybridization (ISH). All pathologic evaluation was performed locally. Prognostic and predictive power of HER2 were assessed with Cox models.
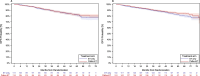
Results: From the original PALLAS intention-to-treat population (N = 5753), 5304 patients (92.2%) were included in this analysis. Among these, 2254 patients (42.5%) were classified as having HER2 IHC 0 (HER2-0), and 3050 (57.5%) as having HER2-low disease (1838 with IHC 1 + and 1212 with IHC 2 +). Median follow-up was 59.8 months. HER2-low prevalence varied significantly across 21 participating countries (range 16.7% to 75.6%; p < 0.001) and was more frequent in patients enrolled in North America (63.1%) than in Europe (53.4%) or other regions (53.4%) (p < 0.001). HER2 status was not significantly associated with iDFS in a multivariable Cox model (hazard ratio 0.93, 95% confidence interval 0.81 - 1.06). No significant interaction was observed between treatment arm and HER2 status for iDFS (p = 0.43). Similar results were obtained for DRFS and OS.
Conclusions: In this large, prospective, global patient cohort, no differences were observed in clinical parameters, prognosis, or differential benefit from palbociclib between HER2-0 and HER2-low tumors. Significant geographic variability was observed in the prevalence of HER2-low status, suggesting a high degree of variation in pathologic assessment of HER2 expression without impact on outcomes.
Keywords: Antibody–drug conjugates; Breast cancer; Endocrine therapy; HER2-low; Palbociclib.
© 2024. The Author(s).
Conflict of interest statement
GN-M reports travel support from AstraZeneca. CS has served as a consultant for Novartis and AstraZeneca; and received grant support from Daiichi-Sankyo and Amgen. DH reports research grants from Pfizer to the institution. AD received institutional research support from Novartis, Pfizer, Genentech, and Neogenomics; and reports that her spouse received support from Pfizer DSMB for a non-oncology trial. PT received institutional research support from AstraZeneca; and served in a consultant or advisory role for AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, Roche, Genentech, Menarini/Stemline, and Novartis. EdA received honoraria from, and/or served on the advisory boards of, Roche/GNE, Novartis, Seagen, Zodiac, Libbs, Pierre Fabre, Lilly, Astra-Zeneca, MSD, and Gilead Sciences; received travel grants from Roche/GNE and AstraZeneca; received a research grant to his institution from Roche/GNE, AstraZeneca, GSK/Novartis, and Gilead Sciences; and served as ESMO director of Membership from 2023–2025 and BSMO President from 2023–2026. GP received honoraria and grants from Pfizer, AstraZeneca, Daiichi-Sankyo, Novartis, Amgen, Roche, Seagen, Accord, and MSD. MM received research grants from Roche, PUMA and Novartis; consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, Taiho Oncology, Daiichi Sankyo, Menarini/Stemline, and Pfizer; and speakers’ honoraria from AstraZeneca, Lilly, Amgen, Roche/Genentech, Novartis, and Pfizer. JMB receives research support from Genentech/Roche and Incyte Corporation; has received advisory board payments from AstraZeneca and Mallinckrodt; and is an inventor on patents regarding immunotherapy targets and biomarkers in cancer. MB served in a consulting or advisory role for Amgen, AstraZeneca, Daiichi-Sankyo/AstraZeneca, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Samsung, Gilead Sciences, and Seagen; served on the Speakers’ Bureaus of Amgen, AstraZeneca, Daiichi-Sankyo/AstraZeneca, Lilly, Novartis, Pierre Fabre, Pfizer, Roche, and Seagen; received institutional research funding from Lilly, Novartis, Pfizer, and Pierre Fabre; and received support for travel, accommodations and expenses from MSD. AMB served as a consultant for AstraZeneca, Pfizer, Novartis, Lilly, Genentech/Roche, Seagen, Daiichi-Sankyo, Merck, Agendia, Sanofi, Puma, Myriad, Gilead, Epic Biosciences, Blueprint, Caris, and Tempus. PGM received honoraria from Pfizer, AstraZeneca, Genomic Health, Novartis, Seagen, Gilead Sciences, and Roche; played a consulting or advisory role for Novartis; received research funding from Teva and Genomic Health (to the institution); and received support for travel, accommodations and expenses from Roche/Genentech. TCH received institutional grant funding from Takeda Oncology; and consulting fees to the institution from Puma Biotechnology. SL received grants and other from Abbvie, AstraZeneca, Celgene, Daiichi-Sankyo, Immunomedics/Gilead, Novartis, and Pfizer; other from Amgen, BMS, EirGenix, Eisai Europe Ltd, GSK, Lilly, Merck KG, Relay Therapeutics, Sanofi, and Olema Pharmaceutics (outside the submitted work); grants from Molecular Health; grants, non-financial support and other from Roche; non-financial support and other from Seagen, other from Olema Pharmaceutics, outside the submitted work; and has a patent VM Scope with royalties paid, a patent EP14153692.0 pending, a patent EP21152186.9 pending, and a patent EP15702464.7 pending. YL is a Pfizer employee and owns Pfizer stocks. LS and CF report research grants from Pfizer to the institution. ELM reports a consulting or advisory role for Lilly, Novartis, and AstraZeneca; and is an associate editor for Breast Cancer Research. MG reports personal fees/travel support from Amgen, AstraZeneca, Daiichi-Sankyo, Eli Lilly, EPG Health (IQVIA), Menarini-Stemline, MSD, Novartis, Pierre Fabre, and Veracyte; and an immediate family member who is employed by Sandoz. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Aromatase inhibitors versus tamoxifen in early breast cancer. patient-level meta-analysis of the randomised trials. Lancet Lond Engl. 2015;386(10001):1341–52. - PubMed
-
- Curigliano G, Burstein HJ, Gnant M, Loibl S, Cameron D, Regan MM, et al. Understanding breast cancer complexity to improve patient outcomes: The St. Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann Oncol [Internet]. 2023 Sep 6 [cited 2023 Sep 7];0(0) - PubMed
-
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–50. - PubMed
-
- Finn RS, Rugo HS, Dieras VC, Harbeck N, Im SA, Gelmon KA, et al. Overall survival (OS) with first-line palbociclib plus letrozole (pal+let) versus placebo plus letrozole (PbO+let) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/her2−ABC): Analyses from PALOMA-2. J Clin Oncol. 2022, 40: 003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

