CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation
- PMID: 39375864
- PMCID: PMC11494708
- DOI: 10.1021/acssynbio.4c00473
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation
Abstract
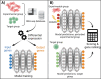
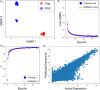
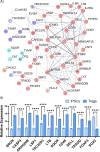
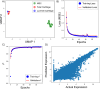
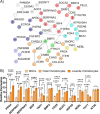
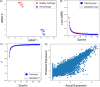
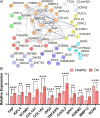
CRISPR gene editing strategies are shaping cell therapies through precise and tunable control over gene expression. However, limitations in safely delivering high quantities of CRISPR machinery demand careful target gene selection to achieve reliable therapeutic effects. Informed target gene selection requires a thorough understanding of the involvement of target genes in gene regulatory networks (GRNs) and thus their impact on cell phenotype. Effective decoding of these complex networks has been achieved using machine learning models, but current techniques are limited to single cell types and focus mainly on transcription factors, limiting their applicability to CRISPR strategies. To address this, we present CRISPR-GEM, a multilayer perceptron (MLP) based synthetic GRN constructed to accurately predict the downstream effects of CRISPR gene editing. First, input and output nodes are identified as differentially expressed genes between defined experimental and target cell/tissue types, respectively. Then, MLP training learns regulatory relationships in a black-box approach allowing accurate prediction of output gene expression using only input gene expression. Finally, CRISPR-mimetic perturbations are made to each input gene individually, and the resulting model predictions are compared to those for the target group to score and assess each input gene as a CRISPR candidate. The top scoring genes provided by CRISPR-GEM therefore best modulate experimental group GRNs to motivate transcriptomic shifts toward a target group phenotype. This machine learning model is the first of its kind for predicting optimal CRISPR target genes and serves as a powerful tool for enhanced CRISPR strategies across a range of cell therapies.
Keywords: CRISPR gene editing; MSC chondrogenesis; gene regulatory network; machine learning; osteoarthritis; regulatory T cell.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Update of
-
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation.bioRxiv [Preprint]. 2024 Jul 3:2024.07.01.601587. doi: 10.1101/2024.07.01.601587. bioRxiv. 2024. Update in: ACS Synth Biol. 2024 Oct 18;13(10):3413-3429. doi: 10.1021/acssynbio.4c00473. PMID: 39005295 Free PMC article. Updated. Preprint.
Similar articles
-
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation.bioRxiv [Preprint]. 2024 Jul 3:2024.07.01.601587. doi: 10.1101/2024.07.01.601587. bioRxiv. 2024. Update in: ACS Synth Biol. 2024 Oct 18;13(10):3413-3429. doi: 10.1021/acssynbio.4c00473. PMID: 39005295 Free PMC article. Updated. Preprint.
-
Prediction of off-target specificity and cell-specific fitness of CRISPR-Cas System using attention boosted deep learning and network-based gene feature.PLoS Comput Biol. 2019 Oct 28;15(10):e1007480. doi: 10.1371/journal.pcbi.1007480. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31658261 Free PMC article.
-
SeqCor: correct the effect of guide RNA sequences in clustered regularly interspaced short palindromic repeats/Cas9 screening by machine learning algorithm.J Genet Genomics. 2020 Nov 20;47(11):672-680. doi: 10.1016/j.jgg.2020.10.007. Epub 2020 Nov 28. J Genet Genomics. 2020. PMID: 33451939
-
Gene Editing With CRISPR/Cas9 RNA-Directed Nuclease.Circ Res. 2017 Mar 3;120(5):876-894. doi: 10.1161/CIRCRESAHA.116.309727. Circ Res. 2017. PMID: 28254804 Review.
-
Rational designs of in vivo CRISPR-Cas delivery systems.Adv Drug Deliv Rev. 2021 Jan;168:3-29. doi: 10.1016/j.addr.2019.11.005. Epub 2019 Nov 21. Adv Drug Deliv Rev. 2021. PMID: 31759123 Review.
References
-
- Graham J.; Werba L.; Federico I.; Gonzalez-Fernandez T. CRISPR Strategies for Stem Cell Engineering: A New Frontier in Musculoskeletal Regeneration. Eur. Cell. Mater. 2023, 46, 91–118. 10.22203/eCM.v046a05. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

