Microneedles with Implantable Tip-Accumulated Therapeutics for the Long-Term Management of Psoriasis
- PMID: 39375985
- PMCID: PMC11657035
- DOI: 10.1002/smll.202405927
Microneedles with Implantable Tip-Accumulated Therapeutics for the Long-Term Management of Psoriasis
Abstract
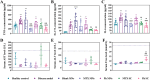
Methotrexate is successfully used as the gold standard for managing moderate-to-severe psoriasis. However, the low bioavailability and short half-life of the oral pills and the invasiveness of the parenteral injections make these suboptimal therapeutic options. Microneedles, bridging the advantages of the former forms, are successfully used to deliver methotrexate for different therapeutic purposes. However, the utilized dissolving microneedles demand frequent administration, potentially compromising patients' compliance. Additionally, the high toxicity of methotrexate prompts a quest for safer alternatives. Phloretin, a natural compound with confirmed antipsoriatic potential, emerges as a promising candidate. Herein, microneedle patches with separable, slow-degrading tips are developed for the sustained delivery of methotrexate and phloretin, as a comprehensive solution for long-term psoriasis management. Both compounds are individually loaded at varying doses and display sustained-release profiles. The developed microneedle patches demonstrate high mechanical strength, favorable drug delivery efficiency, and remarkable antipsoriatic potential both in vitro in keratinocytes and in vivo in a psoriasis mouse model. Comparative analysis with two subcutaneous injections reveals a similar antipsoriatic efficacy with a single patch of either compound, with prominent phloretin safety. Therefore, the developed patches present a superior alternative to methotrexate's current marketed forms and provide a viable alternative (phloretin) with comparable antipsoriatic efficacy and higher safety.
Keywords: PLGA; methotrexate; microneedles; phloretin; psoriasis; sustained release.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
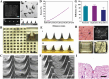

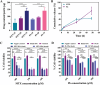
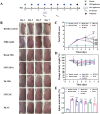
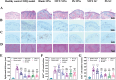
References
-
- Griffiths C. E., Barker J. N., Lancet 2007, 370, 263. - PubMed
-
- a) Chen Z., Br. J. Dermatol. 2018, 179, 16908;
- b) Law J. H., Koo B., Koo J. Y., Psoriasis Forum 2008, 14, 17.
-
- Cronstein B. N., Aune T. M., Nat. Rev. Rheumatol. 2020, 16, 145. - PubMed
-
- Negrei C., Boda D., An Interdisciplinary Approach to Psoriasis, 2017, IntechOpen, London, p. 211.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

