Diagnostic accuracy of exhaled nitric oxide for the non-invasive identification of patients with fibrotic metabolic dysfunction-associated steatohepatitis
- PMID: 39376063
- PMCID: PMC11463020
- DOI: 10.1080/07853890.2024.2410408
Diagnostic accuracy of exhaled nitric oxide for the non-invasive identification of patients with fibrotic metabolic dysfunction-associated steatohepatitis
Abstract
Background: Fibrotic metabolic dysfunction-associated steatohepatitis (MASH) is a condition at risk of progressing to advanced liver disease. We examined whether an innovative exhaled nitric oxide (eNO) breath test (BT) can accurately diagnose fibrotic MASH without requiring blood tests.
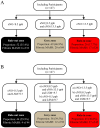
Methods: One hundred and forty-seven patients with MASH were recruited, and all tests were undertaken within 1 week of recruitment. With fibrotic MASH (NAS ≥ 4 and fibrosis stage ≥ 2) as the main outcome indicator, the diagnostic efficacy of eNO in identifying fibrotic MASH was compared to other validated models for advanced fibrosis requiring venesection, namely FAST, Agile 3+, and FIB-4 scores.
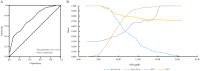
Results: The mean age was 40.36 ± 12.28 years, 73.5% were men. Mean body mass index was 28.83 ± 4.31 kg/m2. The proportion of fibrotic MASH was 29.25%. The area under the receiver operating curve for eNO in diagnosing fibrotic MASH was 0.737 [95% CI 0.650-0.823], which was comparable to FAST (0.751 [0.656-0.846]), Agile 3+ (0.764 [0.670-0.858]), and FIB-4 (0.721 [0.620-0.821]) (all DeLong test p > 0.05). A cut-off of eNO <8.5 ppb gave a sensitivity of 86.0% and a negative predictive value of 88.5% for ruling-out fibrotic MASH. A cut-off of eNO >13.5 ppb provided a specificity of 91.3% and a positive predictive value of 65.4% for ruling-in fibrotic MASH. Sensitivity analyses demonstrated that the diagnostic efficacy of eNO was similar across characteristics such as age. Moreover, adding vibration-controlled transient elastography-LSM (liver stiffness measurement) reduced the uncertainty interval from 46.9% to 39.5%.
Conclusions: The eNO-BT is a promising simple test for non-invasively identifying fibrotic MASH, and its performance is further improved by adding LSM measurement.
Keywords: Exhaled nitric oxide; breath test; fibrotic MASH.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
