HFIP as a versatile solvent in resorcin[ n]arene synthesis
- PMID: 39376488
- PMCID: PMC11457071
- DOI: 10.3762/bjoc.20.211
HFIP as a versatile solvent in resorcin[ n]arene synthesis
Abstract
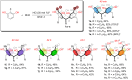
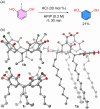
Herein, we present 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as an efficient solvent for synthesizing resorcin[n]arenes in the presence of catalytic amounts of HCl at ambient temperature and within minutes. Remarkably, resorcinols with electron-withdrawing groups and halogens, which are reported in the literature as the most challenging precursors in this cyclization, are tolerated. This method leads to a variety of 2-substituted resorcin[n]arenes in a single synthetic step with isolated yields up to 98%.
Keywords: HFIP; cavitand; cyclization; hydroxyalkylation; resorcinarenes.
Copyright © 2024, Khosravi et al.
Figures

References
-
- Michael A. Am Chem J. 1883;5:338–349.
-
- Michael A, Comey A M. Am Chem J. 1883;5:349–353.
-
- Sen R N, Sinha N N. J Am Chem Soc. 1923;45:2984–2996. doi: 10.1021/ja01665a026. - DOI
-
- Niederl J B, Vogel H J. J Am Chem Soc. 1940;62:2512–2514. doi: 10.1021/ja01866a067. - DOI
-
- Hoegberg A G S. J Org Chem. 1980;45:4498–4500. doi: 10.1021/jo01310a046. - DOI
LinkOut - more resources
Full Text Sources
