Adverse event profiles of CDK4/6 inhibitors: data mining and disproportionality analysis of the FDA adverse event reporting system
- PMID: 39376495
- PMCID: PMC11457275
- DOI: 10.1177/20420986241278498
Adverse event profiles of CDK4/6 inhibitors: data mining and disproportionality analysis of the FDA adverse event reporting system
Abstract
Background: Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors are targeted therapies designed to selectively block CDK4/6, crucial regulators of the cell cycle. These inhibitors play a pivotal role in restoring cell cycle control, particularly in breast cancer cases marked by abnormal CDK regulation, ultimately inhibiting uncontrolled cell division and tumor growth.
Objectives: This analysis aimed to comprehensively examine adverse effects in CDK4/6 inhibitors using the United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database.
Design: Disproportionality analysis was conducted to analyze the adverse event (AE) reports related to CDK4/6 inhibitor submitted to the FAERS database.
Methods: We collected AE reports regarding palbociclib, ribociclib, abemaciclib, trilaciclib, and dalpiciclib submitted to the FAERS from 2015Q1 to 2023Q1. We used the system organ class and the Standardized MedDRA Query to perform a comprehensive search for AEs at the preferred term (PT) level, using case reports as our data source. After removing duplicate reports, we performed disproportionality analysis and sensitivity analysis to identify safety signals.
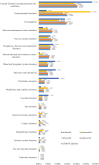
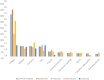
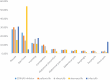
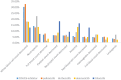
Results: A total of 85,635 reports encompassing 280,211 AEs were extracted for analysis. Among 3681 scrutinized PTs, approximately 484 were detected as statistically significant signals associated with CDK4/6 inhibitors. It was noteworthy that palbociclib and ribociclib had comparable safety profiles, whereas abemaciclib exhibited distinctive safety patterns. Notably, our analysis found novel safety signals linked to CDK4/6 inhibitors, including nail-related disorders such as onychoclasis, nail disorder, and nail discoloration, and psychiatric concerns, including eating disorders and emotional disorder.
Conclusion: Overall, the present study identified several new safety signals of CDK4/6 inhibitors, as well as differences among various drugs within the CDK4/6 category, through the use of the FDA FAERS, which deserve more careful monitoring in the clinic.
Keywords: CDK4/6 inhibitor; FAERS; drug safety; pharmacovigilance; signal.
Plain language summary
A study on the adverse effects of CDK4/6 inhibitors.
Introduction: An adverse event (AE) refers to any undesirable or harmful occurrence that happens to an individual during or after the use of a medical product or intervention. These events are typically reported to regulatory authorities, such as the Food and Drug Administration (FDA), to ensure the safety and effectiveness of medical products. The United States Food and Drug Administration Adverse Event Reporting System (FAERS) database plays a pivotal role in identifying these adverse events. Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors are a class of drugs used to treat certain types of cancer by inhibiting the growth and division of cancer cells. This study investigated the safety signals related to CDK4/6 inhibitors, including palbociclib, ribociclib, abemaciclib, and trilaciclib, by using the FAERS database.
Methods: We collected AE reports associated with CDK4/6 inhibitors that were submitted to the FAERS database between the first quarter of 2015 and the first quarter of 2023. Reporting odds ratio (ROR) method was used identify signals of AEs.
Results: 85,635 AE reports were identified, approximately 484 AE terms were identified as positive signals. Palbociclib and ribociclib had similar safety profiles, while abemaciclib showed a unique pattern. Our analysis also revealed previously unreported AEs, including nail-related disorders such as onychoclasis, nail disorder and nail discolouration. Psychiatric concerns such as eating disorders and emotional disorder were also identified.
Conclusion: We discovered important safety concerns related to CDK4/6 inhibitors. Some of these concerns were consistent with previous studies, while nail-related disorders, eating disorder and emotional disorder were new and not mentioned in the drug labels or existing literature. Our findings may help physicians and pharmacists to weigh the risks and benefits of using CDK4/6 inhibitors in clinical practice.
© The Author(s), 2024.
Figures
Similar articles
-
Comparative analysis of adverse events associated with CDK4/6 inhibitors based on FDA's adverse event reporting system: a case control pharmacovigilance study.BMC Pharmacol Toxicol. 2024 Aug 9;25(1):47. doi: 10.1186/s40360-024-00770-6. BMC Pharmacol Toxicol. 2024. PMID: 39123221 Free PMC article.
-
A post-marketing disproportionality analysis of the safety of ribociclib based on the FDA Adverse Event Reporting System.Ther Adv Drug Saf. 2025 Feb 28;16:20420986251324633. doi: 10.1177/20420986251324633. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40026915 Free PMC article.
-
Infection associated with CDK4/6 inhibitors: a pharmacovigilance analysis of the FDA adverse event reporting system database.Front Pharmacol. 2024 Jul 1;15:1371346. doi: 10.3389/fphar.2024.1371346. eCollection 2024. Front Pharmacol. 2024. PMID: 39011505 Free PMC article.
-
Abemaciclib increases the risk of venous thromboembolism in breast cancer: Integrate meta-analysis, pharmacovigilance database analysis, and in vitro validation.Cancer Treat Rev. 2024 Nov;130:102827. doi: 10.1016/j.ctrv.2024.102827. Epub 2024 Sep 7. Cancer Treat Rev. 2024. PMID: 39278067
-
Hepatic adverse events with CDK4/6 inhibitors: a systematic review combining meta-analysis and FAERS database.Expert Opin Drug Saf. 2025 Feb 23:1-11. doi: 10.1080/14740338.2025.2468357. Online ahead of print. Expert Opin Drug Saf. 2025. PMID: 39960238 Review.
Cited by
-
Targeting CDK4/6 in Cancer: Molecular Docking and Cytotoxic Evaluation of Thottea siliquosa Root Extract.Biomedicines. 2025 Jul 7;13(7):1658. doi: 10.3390/biomedicines13071658. Biomedicines. 2025. PMID: 40722732 Free PMC article.
-
Pharmacokinetics, mass balance, and metabolism of [14C]FCN-437c, a selective and potent CDK4/6 inhibitor in humans.Cancer Chemother Pharmacol. 2025 May 26;95(1):57. doi: 10.1007/s00280-025-04779-4. Cancer Chemother Pharmacol. 2025. PMID: 40418395
References
LinkOut - more resources
Full Text Sources

