Increased peritoneal TGF-β1 is associated with ascites-induced NK-cell dysfunction and reduced survival in high-grade epithelial ovarian cancer
- PMID: 39376560
- PMCID: PMC11456434
- DOI: 10.3389/fimmu.2024.1448041
Increased peritoneal TGF-β1 is associated with ascites-induced NK-cell dysfunction and reduced survival in high-grade epithelial ovarian cancer
Abstract
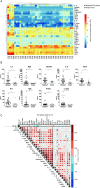
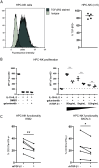
Natural killer (NK) cell therapy represents an attractive immunotherapy approach against recurrent epithelial ovarian cancer (EOC), as EOC is sensitive to NK cell-mediated cytotoxicity. However, NK cell antitumor activity is dampened by suppressive factors in EOC patient ascites. Here, we integrated functional assays, soluble factor analysis, high-dimensional flow cytometry cellular component data and clinical parameters of advanced EOC patients to study the mechanisms of ascites-induced inhibition of NK cells. Using a suppression assay, we found that ascites from EOC patients strongly inhibits peripheral blood-derived NK cells and CD34+ progenitor-derived NK cells, albeit the latter were more resistant. Interestingly, we found that higher ascites-induced NK cell inhibition correlated with reduced progression-free and overall survival in EOC patients. Furthermore, we identified transforming growth factor (TGF)-β1 to correlate with ascites-induced NK cell dysfunction and reduced patient survival. In functional assays, we showed that proliferation and anti-tumor reactivity of CD34+ progenitor-derived NK cells are significantly affected by TGF-β1 exposure. Moreover, inhibition of TGF-β1 signaling with galunisertib partly restored NK cell functionality in some donors. For the cellular components, we showed that the secretome is associated with a different composition of CD45+ cells between ascites of EOC and benign reference samples with higher proportions of macrophages in the EOC patient samples. Furthermore, we revealed that higher TGF-β1 levels are associated with the presence of M2-like macrophages, B cell populations and T-regulatory cells in EOC patient ascites. These findings reveal that targeting TGF-β1 signaling could increase NK cell immune responses in high-grade EOC patients.
Keywords: TGF-β; ascites; natural killer (NK) cells; ovarian cancer; tumor microenvironment.
Copyright © 2024 Maas, Hoogstad-van Evert, Hagemans, Brummelman, van Ens, de Jonge, Hooijmaijers, Mahajan, van der Waart, Hermans, de Klein, Woestenenk, van Herwaarden, Schaap, Rezaeifard, Tauriello, Zusterzeel, Ottevanger, Jansen, Hobo and Dolstra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Puiffe ML, Le Page C, Filali-Mouhim A, Zietarska M, Ouellet V, Tonin PN, et al. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia. (2007) 9(10):820–9. doi: 10.1593/neo.07472 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

