A glimpse into the world of microRNAs and their putative roles in hard ticks
- PMID: 39376631
- PMCID: PMC11456543
- DOI: 10.3389/fcell.2024.1460705
A glimpse into the world of microRNAs and their putative roles in hard ticks
Abstract
Ticks are important blood feeding ectoparasites that transmit pathogens to wildlife, domestic animals, and humans. Hard ticks can feed for several days to weeks, nevertheless they often go undetected. This phenomenon can be explained by a tick's ability to release analgesics, immunosuppressives, anticoagulants, and vasodilators within their saliva. Several studies have identified extracellular vesicles (EVs) as carriers of some of these effector molecules. Further, EVs, and their contents, enhance pathogen transmission, modulate immune responses, and delay wound healing. EVs are double lipid-membrane vesicles that transport intracellular cargo, including microRNAs (miRNAs) to recipient cells. miRNAs are involved in regulating gene expression post-transcriptionally. Interestingly, tick-derived miRNAs have been shown to enhance pathogen transmission and affect vital biological processes such as oviposition, blood digestion, and molting. miRNAs have been found within tick salivary EVs. This review focuses on current knowledge of miRNA loading into EVs and homologies reported in ticks. We also describe findings in tick miRNA profiles, including miRNAs packed within tick salivary EVs. Although no functional studies have been done to investigate the role of EV-derived miRNAs in tick feeding, we discuss the functional characterization of miRNAs in tick biology and pathogen transmission. Lastly, we propose the possible uses of tick miRNAs to develop management tools for tick control and to prevent pathogen transmission. The identification and functional characterization of conserved and tick-specific salivary miRNAs targeting important molecular and immunological pathways within the host could lead to the discovery of new therapeutics for the treatment of tick-borne and non-tick-borne human diseases.
Keywords: RNA transport proteins; extracellular vesicles; miRNA transport; small RNA; tick feeding.
Copyright © 2024 Leal-Galvan, Kumar, Karim, Saelao, Thomas and Oliva Chavez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
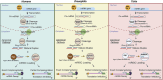

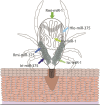
References
-
- Abbott A. L., Alvarez-Saavedra E., Miska E. A., Lau N. C., Bartel D. P., Horvitz H. R., et al. (2005). The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans . Dev. Cell 9 (3), 403–414. 10.1016/j.devcel.2005.07.009 - DOI - PMC - PubMed
-
- Aparicio-Puerta E., Gómez-Martín C., Giannoukakos S., Medina J. M., Scheepbouwer C., García-Moreno A., et al. (2022). sRNAbench and sRNAtoolbox 2022 update: accurate miRNA and sncRNA profiling for model and non-model organisms. Nucleic Acids Res. 50 (W1), W710–w717. 10.1093/nar/gkac363 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

