Enhanced catalytic reduction through in situ synthesized gold nanoparticles embedded in glucosamine/alginate nanocomposites
- PMID: 39376727
- PMCID: PMC11457073
- DOI: 10.3762/bjnano.15.99
Enhanced catalytic reduction through in situ synthesized gold nanoparticles embedded in glucosamine/alginate nanocomposites
Abstract
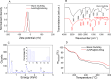
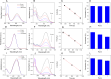
This study introduces a highly efficient and straightforward method for synthesizing gold nanoparticles (AuNPs) within a glucosamine/alginate (GluN/Alg) nanocomposite via an ionotropic gelation mechanism in aqueous environment. The resulting nanocomposite, AuNPs@GluN/Alg, underwent thorough characterization using UV-vis, EDX, FTIR, SEM, TEM, SAED, and XRD analyses. The spherical AuNPs exhibited uniform size with an average diameter of 10.0 nm. The nanocomposites facilitated the recyclable reduction of organic dyes, including 2-nitrophenol, 4-nitrophenol, and methyl orange, employing NaBH4 as the reducing agent. Kinetic studies further underscored the potential of this nanocomposite as a versatile catalyst with promising applications across various industrial sectors.
Keywords: catalysis; gold nanoparticles; organic dyes; organometallic nanocomposites; reduction.
Copyright © 2024, Dang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Sadanandan S, Ramkumar K, Pillai N P, Anuvinda P, Devika V, Ramanunni K, Sreejaya M. Food Control. 2023;148:109510. doi: 10.1016/j.foodcont.2022.109510. - DOI
-
- Santhosh P B, Genova J, Chamati H. Chemistry. 2022;4:345–369. doi: 10.3390/chemistry4020026. - DOI
-
- Elmi G R, Saleem K, Baig M M F A, Aamir M N, Wang M, Gao X, Abbas M, Rehman M U. Magnetochemistry. 2022;8:38. doi: 10.3390/magnetochemistry8040038. - DOI
LinkOut - more resources
Full Text Sources
