Proficiency testing within Eurotransplant
- PMID: 39376741
- PMCID: PMC11456461
- DOI: 10.3389/fgene.2024.1451748
Proficiency testing within Eurotransplant
Abstract
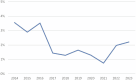
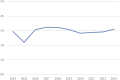
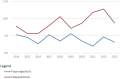
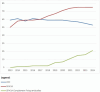
Eurotransplant is responsible for the international allocation of organs between eight countries in Europe. All HLA laboratories affiliated to Eurotransplant must be EFI or ASHI-accredited and must participate in the Eurotransplant external proficiency testing (EPT) program, organized by the Eurotransplant Reference Laboratory (ETRL). EPT within Eurotransplant has a long tradition, starting in 1978. The current EPT program consists of the following schemes: HLA typing including serology, CDC crossmatching, HLA-specific antibody detection, and identification. Participants enter the results of laboratory tests using a web-based application. Assessed results are visible on the website. An additional component called "patient-based cases" runs since 2016. Results are summarized and published on the EPT website. Furthermore, these results are discussed during the annual extramural tissue typers meeting, which is organized by the ETRL. Thanks to this EPT program, the performance of all HLA laboratories affiliated to Eurotransplant can be monitored and corrected, if necessary. Because all affiliated laboratories are assessed in the same EPT program, where these laboratories show to be consistent in most of their results, Eurotransplant EPT has proven to be an efficient tool to create a more uniform level of quality of histocompatibility testing within Eurotransplant.
Keywords: Eurotransplant; histocompatibility testing; patient-based cases; proficiency test; solid organ transplantation.
Copyright © 2024 Zoet, Heidt, van der Linden-van Oevelen, Haasnoot and Claas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Doxiadis I. I., Witvliet M., Verduyn W., de Lange P., Tanke J., Schreuder G. M., et al. (2000). The relevance of proficiency testing for laboratories involved in cadaveric organ transplantation and its consequences for graft survival. Clin. Transpl., 99–103. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

