Probiotics vs. Placebo: Preventing Necrotizing Enterocolitis in a Premature Infant
- PMID: 39376813
- PMCID: PMC11456835
- DOI: 10.7759/cureus.68848
Probiotics vs. Placebo: Preventing Necrotizing Enterocolitis in a Premature Infant
Abstract
Background: Necrotizing enterocolitis (NEC) is a severe gastrointestinal condition primarily affecting preterm newborns, leading to significant morbidity and mortality.
Objective: This prospective cohort study aimed to evaluate the effectiveness of probiotics in preventing NEC in premature infants. Secondary objectives included assessing the impact on mortality, late-onset sepsis, duration of hospital stay, and weight gain.
Methods: The study was conducted at Lady Reading Hospital Medical Teaching Institute, Peshawar, from April 1, 2023, to March 31, 2024, involving 102 preterm infants. Participants were randomly assigned to receive daily oral probiotics (Lactobacillus and Bifidobacterium species) or a placebo.
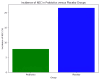
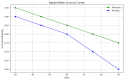
Results: The incidence of NEC was significantly lower in the probiotic group (7.8%) compared to the placebo group (21.6%; p = 0.04). The probiotic group also showed significant reductions in late-onset sepsis (13.7% vs. 29.4%; p = 0.03), shorter hospital stays (42.5 vs. 48.1 days; p = 0.02), and greater weight gain (15.3 vs. 13.4 grams/day; p = 0.01).
Conclusion: These findings support the integration of probiotics into neonatal care, particularly in resource-limited settings.
Keywords: bifidobacterium; lactobacillus; mortality; necrotizing enterocolitis; neonatal care; premature infants; probiotics; prospective cohort study; sepsis.
Copyright © 2024, Ullah et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board (IRB) of Lady Reading Hospital Peshawar issued approval 783/LRH/MTI. The Lady Reading Hospital Medical Teaching Institute Ethical Review Board approved the study, titled "Probiotics vs. Placebo: Preventing Necrotizing Enterocolitis in Premature Infants," under approval number 783/LRH/MTI on March 15, 2023. This approval confirms that the research, conducted by Sami Ullah, Inayatullah Khan, Ayesha, Jabran Ullah Khan, Annam Syed, Fatima Shafiq, and Muhammad Khan of the Department of Pediatrics, MTI/LRH, Peshawar, adhered to all ethical guidelines. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Surgical necrotizing enterocolitis. Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, Blakely ML. https://www.sciencedirect.com/science/article/abs/pii/S0146000516300969?.... Semin Perinatol. 2017;41:70–79. - PMC - PubMed
-
- Unraveling mechanisms of action of probiotics. Sherman PM, Ossa JC, Johnson-Henry K. https://aspenjournals.onlinelibrary.wiley.com/doi/10.1177/0884533608329231. Nutr Clin Pract. 2009;24:10–14. - PubMed
-
- Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Deshpande G, Rao S, Patole S, Bulsara M. Pediatrics. 2010;125:921–930. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous