High-flow humidified oxygen as an early intervention in children with acute severe asthma: a feasibility randomised controlled trial
- PMID: 39377094
- PMCID: PMC11456965
- DOI: 10.1183/23120541.00168-2024
High-flow humidified oxygen as an early intervention in children with acute severe asthma: a feasibility randomised controlled trial
Abstract
Background: Treating children with acute severe asthma (ASA) who fail to respond to first-line inhaled bronchodilators is problematic: use of intravenous agents is inconsistent and side-effects are common. High-flow humidified oxygen (HiFlo) has shown promise in other respiratory conditions and is increasingly used in ASA, but with little evidence.
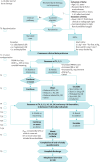
Methods: We conducted a feasibility randomised controlled trial with deferred consent to assess early HiFlo in children aged 2-11 years with ASA not responding to "burst" therapy (high-dose inhaled salbutamol ± ipratropium). Children with Paediatric Respiratory Assessment Measure (PRAM) score 5+ after "burst" were randomised to commence HiFlo or follow standard care. Candidate primary outcomes assessed were treatment failure requiring escalation, and time to meeting hospital discharge criteria.
Results: The target was met despite coronavirus disease 2019 pandemic disruption: 56 children were randomised across four sites, with deferred consent received in 50 out of 56 (89%), and mean recruitment rate 1.1 per site per month. 28 were allocated early HiFlo and 22 standard care. Data collection was complete for both candidate primary outcomes. Treatment failure requiring escalation occurred in 18 of 28 children (64%) in the HiFlo arm and in 19 of 22 (86%) in the standard care arm. Median (interquartile range) time from randomisation to meeting discharge criteria was 29.3 h (21.8-43.7 h) in the HiFlo arm and 36.8 h (24.1-46.3 h) in the standard care arm.
Conclusions: HiFlo in childhood ASA is a potentially promising intervention whose use is increasing despite lack of evidence. A definitive randomised controlled trial to assess its effectiveness is required and appears to be feasible.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: A. Kapur has nothing to disclose. Conflict of interest: H. Rojas-Anaya has nothing to disclose. Conflict of interest: G. Roberts has nothing to disclose. Conflict of interest: D. Roland has nothing to disclose. Conflict of interest: A. Gupta has nothing to disclose. Conflict of interest: M. Lazner was an advisory board member for Aerogen Ltd for non-HiFlo study-related research and received an honorarium of £952.89 for this work. Conflict of interest: J. Bayreuther has nothing to disclose. Conflict of interest: F. Cantle has nothing to disclose. Conflict of interest: C. Jones has nothing to disclose. Conflict of interest: J. Pappachan has nothing to disclose. Conflict of interest: S. Bremner has nothing to disclose. Conflict of interest: D. James has nothing to disclose. Conflict of interest: S. Fitzgerald has nothing to disclose. Conflict of interest: K. Owens has nothing to disclose. Conflict of interest: L. Asim has nothing to disclose. Conflict of interest: E. Khaleva has nothing to disclose. Conflict of interest: P. Seddon, in March 2023, chaired an advisory board (unrelated to HiFlo) for Aerogen Ltd, which loaned nebuliser equipment for the study, and received an honorarium.
Figures


References
-
- Merrill C, Owens PL. Reasons for being admitted to the hospital through the emergency department for children and adolescents, 2004: Statistical Brief #33. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, Agency for Healthcare Research and Quality, 2007. www.ncbi.nlm.nih.gov/books/NBK61976/ - PubMed
-
- Sinha I, Latchem S, Amusan L, et al. National Asthma and COPD Audit Programme: Children and young people asthma clinical and organisational audit 2019/20. Combined clinical and organisational audit of children and young people asthma services in England, Scotland and Wales. London, Royal College of Physicians, 2021. www.nrap.org.uk/NRAP/welcome.nsf/0/FD67A41147AF27E4802586D80055CC2F/$fil...
-
- British Thoracic Society ( BTS)/Scottish Intercollegiate Guidelines Network (SIGN). SIGN 158: British Guideline on the Management of Asthma. 2019. www.sign.ac.uk/media/1773/sign158-updated.pdf
LinkOut - more resources
Full Text Sources
Miscellaneous
