Defining Cardiomyocyte Repolarization Response to Pharmacotherapy in Long-QT Syndrome Type 3
- PMID: 39377211
- PMCID: PMC11935574
- DOI: 10.1161/JAHA.124.034690
Defining Cardiomyocyte Repolarization Response to Pharmacotherapy in Long-QT Syndrome Type 3
Abstract
Background: Long-QT syndrome is a primary cardiac ion channelopathy predisposing a patient to ventricular arrhythmia through delayed repolarization on the resting ECG. We aimed to establish a patient-specific, human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes model of long-QT syndrome type 3 (LQT3) using clustered regularly interspaced palindromic repeats (CRISPR/Cas9), for disease modeling and drug challenge.
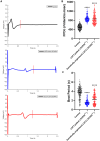
Methods and results: HiPSCs were generated from a patient with LQT3 harboring an SCN5A pathogenic variant (c.1231G>A; p.Val411Met), and an unrelated healthy control. The same SCN5A pathogenic variant was engineered into the background healthy control hiPSCs via CRISPR/Cas9 gene editing to generate a second disease model of LQT3 for comparison with an isogenic control. All 3 hiPSC lines were differentiated into cardiomyocytes. Both the patient-derived LQT3 (SCN5A+/-) and genetically engineered LQT3 (SCN5A+/-) hiPSC-derived cardiomyocytes showed significantly prolonged cardiomyocyte repolarization compared with the healthy control. Mexiletine, a cardiac voltage-gated sodium channel (NaV1.5) blocker, shortened repolarization in both patient-derived LQT3 and genetically engineered LQT3 hiPSC-derived cardiomyocytes, but had no effect in the control. Notably, calcium channel blockers nifedipine and verapamil showed a dose-dependent shortening of repolarization, rescuing the phenotype. Additionally, therapeutic drugs known to prolong the corrected QT in humans (ondansetron, clarithromycin, and sotalol) demonstrated this effect in vitro, but the LQT3 clones were not more disproportionately affected compared with the control.
Conclusions: We demonstrated that patient-derived and genetically engineered LQT3 hiPSC-derived cardiomyocytes faithfully recapitulate pathologic characteristics of LQT3. The clinical significance of such an in vitro model is in the exploration of novel therapeutic strategies, stratifying drug adverse reaction risk and potentially facilitating a more targeted, patient-specific approach in high-risk patients with LQT3.
Keywords: CRISPR/Cas9; SCN5A; human induced pluripotent stem cells; long‐QT syndrome; multielectrode array.
Figures


References
-
- Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

