N-Acetyl-L-Cysteine (NAC) Blunts Axitinib-Related Adverse Effects in Preclinical Models of Glioblastoma
- PMID: 39377544
- PMCID: PMC11460215
- DOI: 10.1002/cam4.70279
N-Acetyl-L-Cysteine (NAC) Blunts Axitinib-Related Adverse Effects in Preclinical Models of Glioblastoma
Abstract
Objective: Axitinib is a tyrosine kinase inhibitor characterized by a strong affinity for Vascular Endothelial Growth Factor Receptors (VEGFRs). It was approved in 2012 by Food and Drug Administration and European Medicines Agency as a second line treatment for advanced renal cell carcinoma and is currently under evaluation in clinical trial for the treatment of other cancers. Glioblastoma IDH-wild type (GBM) is a highly malignant brain tumor characterized by diffusely infiltrative growth pattern and by a prominent neo-angiogenesis. In GBM, axitinib has demonstrated a limited effectiveness as a monotherapy, while it was recently shown to significantly improve its efficacy in combination treatments. In preclinical models, axitinib has been reported to trigger cellular senescence both in tumor as well as in normal cells, through a mechanism involving intracellular reactive oxygen species (ROS) accumulation and activation of Ataxia Telangiectasia Mutated kinase (ATM). Limiting axitinib-dependent ROS increase by antioxidants prevents senescence specifically in normal cells, without affecting tumor cells.
Methods: We used brain tumor xenografts obtained by engrafting Glioma Stem Cells (GSCs) into the brain of immunocompromised mice, to investigate the hypothesis that the antioxidant molecule N-Acetyl-L-Cysteine (NAC) might be used to reduce senescence-associated adverse effects of axitinib treatment without altering its anti-tumor activity.
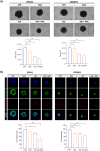
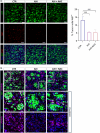
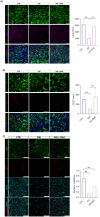
Results: We demonstrate that the use of the antioxidant molecule N-Acetyl-Cysteine (NAC) in combination with axitinib stabilizes tumor microvessels in GBM tumor orthotopic xenografts, eventually resulting in vessel normalization, and protects liver vasculature from axitinib-dependent toxicity.
Conclusion: Overall, we found that NAC co-treatment allows vessel normalization in brain tumor vessels and exerts a protective effect on liver vasculature, therefore minimizing axitinib-dependent toxicity.
Keywords: N‐acetyl‐L‐cysteine; axitinib; brain tumor xenograft; endothelium; glioblastoma IDH‐wild type; glioma stem cells; therapy; toxicity.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Hu‐Lowe D. D., Zou H. Y., Grazzini M. L., et al., “Nonclinical Antiangiogenesis and Antitumor Activities of Axitinib (AG‐013736), an Oral, Potent, and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases 1, 2, 3,” Clinical Cancer Research 14 (2008): 7272–7283. - PubMed
-
- Infante J. R., Reid T. R., Cohn A. L., et al., “Axitinib and/or Bevacizumab With Modified FOLFOX‐6 as First‐Line Therapy for Metastatic Colorectal Cancer: A Randomized Phase 2 Study,” Cancer 119 (2013): 2555–2563. - PubMed
-
- Bendell J. C., Joseph M., Barnes K., et al., “A Phase‐2 Trial of Single Agent Axitinib as Maintenance Therapy Following First‐Line Treatment With Modified FOLFOX/Bevacizumab in Patients With Metastatic Colorectal Cancer,” Cancer Investigation 35 (2017): 386–392. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

