Evaluation of Growth Characteristics and Final Height of Cases Diagnosed with Noonan Syndrome on Growth Hormone Treatment
- PMID: 39377546
- PMCID: PMC11923494
- DOI: 10.4274/jcrpe.galenos.2024.2024-7-3
Evaluation of Growth Characteristics and Final Height of Cases Diagnosed with Noonan Syndrome on Growth Hormone Treatment
Abstract
Objective: Proportional short stature is one of the most important features of Noonan syndrome (NS), and adult height often remains below the third percentile. Although the pathophysiology of short stature in NS patients is not fully understood, it has been shown that growth hormone (GH) treatment is beneficial in NS, significantly improving height in respect to the results of short and long-term GH treatment.
Methods: In this national retrospective cohort study, patients with NS who reached final height from 14 centers were evaluated. Patients were stratified by sex and treatment with or without GH and final height outcomes were compared.
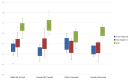
Results: The study included 67 patients with NS, of whom 53 (79.1%) with mean follow-up 5.6 years, received GH treatment. At presentation height standard deviation scores (SDS) of the subjects who were started on GH tended to be shorter than those who did not (-3.26±1.07 vs. -2.53±1.23). In girls mean final height and final height SDS in those using GH vs not using GH were 150.1 cm (-2.17 SDS) vs. 147.4 cm (-2.8 SDS), respectively, and for boys these values were 162.48 cm (-1.81 SDS) vs 157.46 cm (-2.68 SDS), respectively. The Δheight SDS value of the cases was significantly higher in the group receiving GH compared to those not receiving GH (1.36±1.12 SD vs. -0.2±1.24, p<0.001). Cardiac findings remained stable in two patients with hypertrophic cardiomyopathy who received GH treatment. No significant side effects were observed in any patient during follow-up.
Conclusion: In patients with NS who reach their final height, a significant increase in height was observed with GH treatment. An increase of approximately +1.4 SDS may be achieved. GH treatment appears to be safe and effective in NS.
Keywords: Noonan syndrome; Final height; growth hormone; treatment.
©Copyright 2025 by Turkish Society for Pediatric Endocrinology and Diabetes / The Journal of Clinical Research in Pediatric Endocrinology published by Galenos Publishing House.
Conflict of interest statement
Conflict of Interest: Four authors of this article, Serap Turan, Korcan Demir, Abdullah Bereket and Feyza Darendeliler, are a members of the Editorial Board of the Journal of Clinical Research in Pediatric Endocrinology. However, they did not involved in any stage of the editorial decision of the manuscript. The other authors declared no conflict of interest.
Figures
References
-
- De Rocca Serra-Nédélec A, Edouard T, Tréguer K, Tajan M, Araki T, Dance M, Mus M, Montagner A, Tauber M, Salles JP, Valet P, Neel BG, Raynal P, Yart A. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc Natl Acad Sci U S A. 2012;109(11):4257–4262. doi: 10.1073/pnas.1119803109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous