Investigation into the significant role of dermal-epidermal interactions in skin ageing utilising a bioengineered skin construct
- PMID: 39377615
- PMCID: PMC11701872
- DOI: 10.1002/jcp.31463
Investigation into the significant role of dermal-epidermal interactions in skin ageing utilising a bioengineered skin construct
Abstract
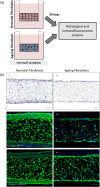
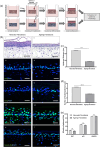
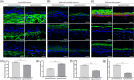
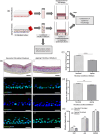
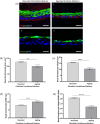
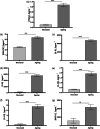
Increased prevalence of skin ageing is a growing concern due to an ageing global population and has both sociological and psychological implications. The use of more clinically predictive in vitro methods for dermatological research is becoming commonplace due to initiatives and the cost of clinical testing. In this study, we utilise a well-defined and characterised bioengineered skin construct as a tool to investigate the cellular and molecular dynamics involved in skin ageing from a dermal perspective. Through incorporation of ageing fibroblasts into the dermal compartment we demonstrate the significant impact of dermal-epidermal crosstalk on the overlying epidermal epithelium. We characterise the paracrine nature of dermal-epidermal communication and the impact this has during skin ageing. Soluble factors, such as inflammatory cytokines released as a consequence of senescence associated secretory phenotype (SASP) from ageing fibroblasts, are known to play a pivotal role in skin ageing. Here, we demonstrate their effect on epidermal morphology and thickness, but not keratinocyte differentiation or tissue structure. Through a novel in vitro strategy utilising bioengineered tissue constructs, this study offers a unique reductionist approach to study epidermal and dermal compartments in isolation and tandem.
Keywords: ageing; bioengineering; dermal‐epidermal crosstalk; extracellular matrix; human skin equivalent; inflammation.
© 2025 The Author(s). Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
Ben Hulette, Teresa Dicolandrea, Robert Isfort, John Oblong, Michael J.Flagler, Charlie Bascom were full‐time employees of Procter & Gamble (Cincinnati, OH, USA) at the time this study was conducted. This work was supported by funding from The Procter & Gamble Company. Stefan Przyborski collaborates and acts as a technical consultant for the company Reprocell Europe Ltd. All other authors declare no conflicts of interest.
Figures
References
-
- Admane, P. , Gupta, A. C. , Jois, P. , Roy, S. , Chandrasekharan Lakshmanan, C. , Kalsi, G. , Bandyopadhyay, B. , & Ghosh, S. (2019). Direct 3D bioprinted full‐thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting, 15, e00051. 10.1016/j.bprint.2019.e00051 - DOI
-
- Allcock, B. , Wei, W. , Goncalves, K. , Hoyle, H. , Robert, A. , Quelch‐Cliffe, R. , Hayward, A. , Cooper, J. , & Przyborski, S. (2023). Impact of the physical cellular microenvironment on the structure and function of a model hepatocyte cell line for drug toxicity applications. Cells, 12(Issue 19), 2408. 10.3390/cells12192408 - DOI - PMC - PubMed
-
- Bjerke, D. L. , Li, R. , Price, J. M. , Dobson, R. L. M. , Rodrigues, M. , Tey, C. , Vires, L. , Adams, R. L. , Sherrill, J. D. , Styczynski, P. B. , Goncalves, K. , Maltman, V. , Przyborski, S. , & Oblong, J. E. (2021). The vitamin A ester retinyl propionate has a unique metabolic profile and higher retinoid‐related bioactivity over retinol and retinyl palmitate in human skin models. Experimental Dermatology, 30(2), 226–236. 10.1111/exd.14219 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

