IOP Reduction in Nonhuman Primates by Microneedle Injection of Drug-Free Hydrogel to Expand the Suprachoroidal Space
- PMID: 39377753
- PMCID: PMC11469220
- DOI: 10.1167/tvst.13.10.14
IOP Reduction in Nonhuman Primates by Microneedle Injection of Drug-Free Hydrogel to Expand the Suprachoroidal Space
Abstract
Purpose: Expansion of the suprachoroidal space (SCS) by a hydrogel injection has been shown to reduce intraocular pressure (IOP) in rabbits as a potential treatment for ocular hypertension in glaucoma. Here, we evaluate the safety and efficacy of this approach in hypertensive and normotensive eyes in nonhuman primates.
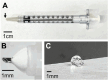
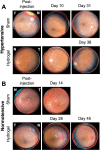
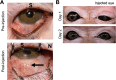
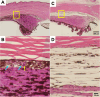
Methods: A microneedle was used to inject a hyaluronic acid-based hydrogel or saline solution (control) into the SCS of cynomolgus monkey eyes that were either normotensive (n = 7 experimental; n = 2 control eyes) or had induced ocular hypertension (n = 6 experimental; n = 3 control eyes). IOP and the degree of SCS expansion were monitored over time by tonometry and ultrasound biomicroscopy, respectively. Safety was evaluated through slit lamp, fundus, and histology examinations.
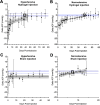
Results: In hypertensive eyes, SCS injection with hydrogel initially reduced IOP by 47.5 ± 16.7%, and IOP returned to baseline in 38 days. In normotensive eyes, hydrogel injection initially reduced IOP by 38.8 ± 8.1% and IOP gradually returned to baseline also in 39 days. Sham injections resulted in mild IOP reduction in hypertensive eyes and normotensive eyes. The hydrogel injections were well tolerated by clinical assessments.
Conclusions: IOP was reduced in nonhuman primates for over one month by sustained SCS expansion. This procedure was safe and simple to perform. These data confirm the translational potential of this treatment method. Further optimization of the hydrogel may provide longer durations of IOP reduction.
Translational relevance: A microneedle injection of hydrogel into the suprachoroidal space may provide a non-surgical, non-pharmacologic treatment for ocular hypertension in glaucoma patients.
Conflict of interest statement
Disclosure:
Figures



References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. - PubMed
-
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998; 126: 498–505. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. .. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol (Chicago, Ill 1960). 2002; 120: 701–713; discussion 829–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

