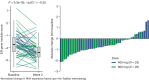
A Preoperative Window-of-Opportunity Study of Oral SERD, Imlunestrant, in Newly Diagnosed ER-Positive, HER2-Negative Early Breast Cancer: Results from the EMBER-2 Study
- PMID: 39377773
- PMCID: PMC11609621
- DOI: 10.1158/1078-0432.CCR-24-2113
A Preoperative Window-of-Opportunity Study of Oral SERD, Imlunestrant, in Newly Diagnosed ER-Positive, HER2-Negative Early Breast Cancer: Results from the EMBER-2 Study
Abstract
Purpose: Imlunestrant is an oral selective estrogen receptor degrader with favorable safety and preliminary efficacy in patients with advanced breast cancer. Pharmacodynamic (PD) biomarker data can optimize drug dosing; in this study, we present PD data from the EMBER-2 study.
Patients and methods: Postmenopausal women with untreated, operable estrogen receptor (ER)-positive, HER2-negative early breast cancer were randomized to 400 versus 800 mg of imlunestrant daily for ∼2 weeks before surgery. A single arm study tested a daily dose of 200 mg. PD biomarker changes (ER, progesterone receptor, Ki-67 by IHC, and mRNA expression of ER-related genes) were evaluated in paired tumor samples (pre-/posttreatment). Safety and pharmacokinetics were also assessed.
Results: Among evaluable paired samples (n = 75), PD profiles demonstrated consistent ER targeting between 400- and 800-mg doses, with less toxicity at the 400-mg dose. Although inducing the lowest rate of complete cell-cycle arrest, PD and pharmacokinetic results were similar for the 200-mg dose.
Conclusions: EMBER-2 combined with existing phase I data has identified 400 mg as the optimal imlunestrant dose.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M. Vidal reports personal fees from Novartis, Daiichi Sankyo, AstraZeneca, Eli Lilly and Company, and Gilead outside the submitted work. M. Martín reports grants and personal fees from Novartis; grants, personal fees, and other support from Roche; personal fees from Eli Lilly and Company, Daiichi Sankyo, and Pfizer; grants from Puma, and personal fees and other support from AstraZeneca outside the submitted work. P.A. Kaufman reports grants from the University of Vermont Cancer Center during the conduct of the study, personal fees from Eli Lilly and Company, and grants from the University of Vermont Cancer Center outside the submitted work. N. Harbeck reports personal fees from AstraZeneca, Daiichi Sankyo, Gilead, Eli Lilly and Company, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Viatris, and Zuellig Pharma outside the submitted work, as well as minority ownership at West German Study Group. K.K. Hunt reports grants from Eli Lilly and Company during the conduct of the study, personal fees from Armada Health and AstraZeneca, and grants from CairnSurgical and Lumicell outside the submitted work. S. Carter reports nonfinancial support and other support from Eli Lilly and Company during the conduct of the study. F.-C. Bidard reports grants, personal fees, and nonfinancial support from AstraZeneca and Pfizer and personal fees from Roche and Eli Lilly and Company outside the submitted work. P.A. Fasching reports personal fees from Eli Lilly and Company during the conduct of the study; grants from BioNTech and Cepheid; grants and personal fees from Pfizer; and personal fees from Novartis, Daiichi Sankyo, AstraZeneca, Eisai, Merck Sharp & Dohme, Seagen, Roche, Agendia, Gilead, Mylan, Menarini Stemline, Veracyte, and Guardant Health outside the submitted work. P. Aftimos reports grants and personal fees from Roche, personal fees from Boehringer Ingelheim, MacroGenics, Novartis, Amcure, Servier, G1 Therapeutics, Radius, Deloitte, Synthon, Eli Lilly and Company, and Menarini; personal fees and other support from Daiichi Sankyo, Gilead, and Amgen; and other support from MSD and Pfizer outside the submitted work. D. Wheatley reports consulting fees from AstraZeneca, Gilead, Novartis, and Roche. E. Hamilton reports grants and other support from Eli Lilly and Company during the conduct of the study; grants and other support from Accutar Biotech, Arvinas, AstraZeneca, Daiichi Sankyo, Gilead Sciences, Eli Lilly and Company, Mersana, Olema, Pfizer, Roche/Genentech, and Stemline Therapeutics; grants from AbbVie, Acerta Pharma, ADC Therapeutics, Akeso Bio Australia, Amgen, Aravive, ArQule, ARTIOS, Atlas Medx, BeiGene, Black Diamond, Bliss BioPharmaceutical, Boehringer Ingelheim, Bristol Myers Squibb, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan, Curis, CytomX, Dana-Farber Cancer Institute, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Eisai, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon Pharmaceuticals, Fujifilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchison MediPharma, ImmunoGen, Immunomedics, Incyte, Infinity Pharmaceuticals, Inspirna, InvestisBio, Jacobio, Karyopharm, K-GROUP BETA, Kind Pharmaceuticals, Leap Therapeutics, Loxo Oncology, Lycera, MabSpace Biosciences, MacroGenics, MedImmune, Merus, Millennium, Molecular Templates, Myriad Genetic Laboratories, Novartis, NuCana, OncoMed, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Prelude Therapeutics, ProfoundBio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicines, Rgenix, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback Therapeutics, Stemcentrx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics, and Zymeworks; and other support from Circle Pharma, Entos, IQVIA, Janssen, Jazz Pharmaceuticals, Jefferies LLC, Johnson and Johnson, Medical Pharma Services, Shorla Pharma, Tempus Labs, Theratechnologies, Tubulis, and Zentalis Pharmaceuticals outside the submitted work. P. Schmid reports grants and personal fees from AstraZeneca, Merck, Novartis, and Roche; personal fees from Pfizer, Bayer, Puma, Boehringer Ingelheim, Eisai, and Celgene; and grants from Astellas, Genentech, OncoGenex, and Medivation outside the submitted work. M. Bhave reports other support from Merck and Pfizer and grants and other support from Eli Lilly and Company, AstraZeneca, and Daiichi Sankyo outside the submitted work. R. Ismail-Khan reports employment with Eli Lilly and Company and ownership of Eli Lilly and Company stocks. C. Karacsonyi reports other support from Eli and Lilly Company during the conduct of the study, as well as other support from Eli Lilly and Company outside the submitted work. S.T. Estrem reports other support from Eli Lilly and Company during the conduct of the study. B. Nguyen reports employment with Eli Lilly and Company and being an equity holder in Eli Lilly and Company. U. Ozbek reports other support from Eli Lilly and Company during the conduct of the study. E. Yuen reports personal fees from Eli Lilly and Company during the conduct of the study. V. Rodrik-Outmezguine reports other support from Eli Lilly and Company during the conduct of the study, as well as other support from Eli Lilly and Company outside the submitted work. E. Ciruelos reports personal fees from Eli Lilly and Company, Roche, Pfizer, Gilead, and AstraZeneca outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- NCCN . NCCN clinical practice guidelines in oncology (NCCN Guidelines). Breast cancer. Version 1.2024. 2024. [cited 2024 Feb 21]. Available from:https://www.nccn.org/professionals/physician_gls/default.aspx.
-
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. - PubMed
-
- Gombos A. Selective oestrogen receptor degraders in breast cancer: a review and perspectives. Curr Opin Oncol 2019;31:424–9. - PubMed
-
- Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2024;35:159–82. - PubMed
-
- Robertson JF, Erikstein B, Osborne KC, Pippen J, Come SE, Parker LM, et al. Pharmacokinetic profile of intramuscular fulvestrant in advanced breast cancer. Clin Pharmacokinet 2004;43:529–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous