Pharmacokinetic analysis and simplified uptake measures for tumour lesion [18F]F-AraG PET imaging in patients with non-small cell lung cancer
- PMID: 39377810
- PMCID: PMC11732896
- DOI: 10.1007/s00259-024-06931-3
Pharmacokinetic analysis and simplified uptake measures for tumour lesion [18F]F-AraG PET imaging in patients with non-small cell lung cancer
Abstract
Introduction: The novel positron emission tomography (PET) imaging tracer, [18F]F-AraG, targets activated T-cells, offering a potential means to improve our understanding of immune-oncological processes. The aim of this study was to determine the optimal pharmacokinetic model to quantify tumour lesion [18F]F-AraG uptake in patients with non-small cell lung cancer (NSCLC), and to validate simplified measures at different time intervals against the pharmacokinetic uptake parameter.
Methods: Ten patients with early-stage NSCLC and three patients with advanced NSCLC underwent a dynamic PET scan of minimal 60 min. Venous and/or arterial blood sampling was obtained at maximum seven time points. Tumour lesion time activity curves and metabolite-corrected input functions were analysed using single-tissue reversible (1T2k), two-tissue irreversible (2T3k) and two-tissue reversible (2T4k) plasma input models. Simplified uptake measures, such as standardised uptake value (SUV) and tumour-to-blood (TBR) or tumour-to-plasma ratio (TPR), were evaluated for different time intervals.
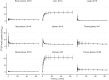
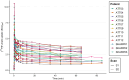
Results: Whole-blood and plasma radioactivity concentrations showed rapid clearance of [18F]F-AraG. Metabolite analysis revealed a low rate of metabolism, at 70 min p.i., on average, 79% (SD = 9.8%) of the total radioactivity found in blood corresponded to intact [18F]F-AraG. The time activity curves were best fitted by the 2T3k model. Strong positive correlations were found for SUV (body weight (BW), lean body mass (LBM) or body surface area (BSA) corrected), TBR and TPR for any time interval between 20 and 70 min p.i. against the 2T3k-derived Ki. The correlation of TBR at 60-70 min p.i. with 2T3K-derived Ki (r (df = 20) = 0.87, p < 0.01), was stronger than for SUVBW (r (df = 20) = 0.80, p < 0.01).
Conclusion: Tumour lesion [18F]F-AraG uptake in patients with NSCLC is characterised by a 2T3k model. TBR and TPR show most potential for simplified quantification of tumour lesion [18F]F-AraG uptake in patients with NSCLC.
Keywords: Activated T-cells; ImmunoPET; Pharmacokinetic modelling; [18F]F-AraG.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Amsterdam University Medical Center. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable. Competing interests: Andrea Thiele is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany. CellSight Technologies (CST) is commercializing [18F]F-AraG. Jelena Levi holds an equity interest in CST and patents related to [18F]F-AraG.
Figures



References
-
- Brahmer J, Rodriguez-Abreu D, Robinson A, Hui R, Csőszi T, Fülöp A, et al. LBA51 KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥ 50%. Ann Oncol. 2020;31:S1181–2. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

