Real-world insights into the management of hemophilia A in Italy: treatment patterns and healthcare resource utilization
- PMID: 39377876
- PMCID: PMC11461399
- DOI: 10.1007/s44313-024-00034-6
Real-world insights into the management of hemophilia A in Italy: treatment patterns and healthcare resource utilization
Abstract
Purpose: This real-world analysis described the Hemophilia A (HA) population in Italy, evaluating drug utilization and consumption of factor VIII (FVIII) products of patients under prophylaxis and on-demand therapy.
Methods: From Jan-2017 to Jun-2022, male patients with HA were identified through prescriptions of FVIII products [extended half-life FVIII, standard half-life recombinant FVIII, and plasma-derived FVIII (EHL FVIII, SHL rFVIII, and pdFVIII, respectively)], or emicizumab or FVIII plus von Willebrand factor or HA-related hospitalization using administrative flows of Italian healthcare entities. Patients on treatment with FVIII products during 2021-2022 were stratified by treatment regimen (prophylaxis/on-demand). The mean annual consumption expressed in International Units (IU) of EHL FVIII and SHL FVIII in patients treated during 2021-2022 having at least 12-month follow-up were assessed.
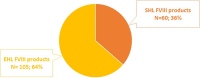
Results: Among included HA patients, 145 (39.5%) received EHL FVIII and 222 (60.5%) SHL FVIII. Of 165 patients on prophylaxis, 105 (64%) received an EHL FVIII and 60 (36%) an SHL FVIII. The mean annual consumption of FVIII was 336,700 IU (median 319,000 IU) for EHL FVIII and 440,267 IU (median 360,500 IU) for SHL FVIII. Specifically, for patients on EHL FVIII, the most common drugs were efmoroctocog alfa (N = 51) and damoctocog alfa pegol (N = 50), followed by turoctocog alfa pegol (N = 25) and rurioctocog alfa pegol (N = 19). Of 702 HA patients initially treated with FVIII products, 74 (10.5%) switched to emicizumab during follow-up.
Conclusion: These findings revealed an extensive use of EHL FVIII products, suggesting growing efforts from clinicians to optimize prophylactic strategies and achieve better bleeding protection.
Keywords: Healthcare resource utilization; Hemophilia A; Monoclonal antibodies; Plasma-derived FVIII; Treatment patterns.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Berntorp E, Fischer K, Hart DP, et al. Haemophilia. Nat Rev Dis Primers. 2021;7(1):45. 10.1038/s41572-021-00278-x. - PubMed
-
- Salen P, Babiker HM. Hemophilia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
-
- World Federation of Hemophilia Report on the Annual Global Survey 2021. Available at: https://www1.wfh.org/publications/files/pdf-2324.pdf, last accessed 07 March, 2024
LinkOut - more resources
Full Text Sources
Miscellaneous