Postpartum Psychiatric Outcomes and Sick Leave After Discontinuing SSRI or SNRI in Pregnancy
- PMID: 39378031
- PMCID: PMC11581648
- DOI: 10.1001/jamanetworkopen.2024.38269
Postpartum Psychiatric Outcomes and Sick Leave After Discontinuing SSRI or SNRI in Pregnancy
Abstract
Importance: Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are consistently reported to be discontinued by approximately half of pregnant women. Little is known about how this may be associated with postpartum psychiatric health.
Objective: To investigate associations of SSRI or SNRI discontinuation in pregnant women with depression or anxiety and psychiatric health and sick leave absence after childbirth.
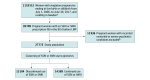
Design, setting, and participants: This population-based cohort study was conducted between 2006 and 2019 using data from Swedish population-based registers. Pregnant women with a filled prescription of an SSRI or SNRI in the 90 days before pregnancy without recorded comorbid or severe psychiatric conditions were included. Analyses were performed in November 2023.
Exposures: K-means for longitudinal data was used to cluster trajectories of SSRI and SNRI use during pregnancy, resulting in 2 trajectory groups based on the number of days covered, defined as continued and discontinued use groups.
Main outcomes and measures: The primary outcome was psychiatric-related hospitalizations by 90 days after childbirth. Secondary outcomes included psychiatric-related outpatient visits, self-harm and suicide, and any-cause mortality by 90 days after childbirth and all outcomes plus sick leave absence by 1.5 years after childbirth.
Results: Among 27 773 pregnant women (17 241 aged ≥30 years [62.1%] at childbirth), 13 184 women (47.5%) had discontinued SSRI or SNRI use and 14 589 individuals (52.5%) had continued use. Individuals in the discontinued compared with continued use group were younger (5588 women [42.4%] vs 4944 women [33.9%] aged <30 years), less educated (4281 women [32.5%] vs 5821 women [39.9%] who completed postsecondary education or above), and more likely to have smoked in early pregnancy (1445 individuals [11.0%] vs 1180 individuals [8.1%]), been born in a non-Nordic country (1641 individuals [12.4%] vs 975 individuals [6.7%]), and used anxiolytics (1301 individuals [9.9%] vs 1119 individuals [7.7%]) and hypnotics and sedatives (1609 individuals [12.2%] vs 1510 individuals [10.4%]). Psychiatric-related hospitalizations occurred in 49 individuals (0.4%) in the discontinued vs 59 individuals (0.5%) in the continued use group in the 90 days after childbirth, with an adjusted hazard ratio (aHR) of 1.28 (95% CI, 0.85-1.91), while at 1.5 years after childbirth, the aHR was 0.81 (95% CI, 0.66-1.00). Lower hazard rates for psychiatric-related outpatient visits in the discontinued vs continued use group at 90 days (aHR, 0.59; 95% CI, 0.53-0.66) and 1.5 years (aHR, 0.60; 95% CI, 0.57-0.64) after childbirth were found. No difference in sick leave absence was found; however, individuals who discontinued had fewer days of sick leave by 1.5 years after childbirth than those who continued (mean [SD], 44.6 [70.6] days vs 53.1 [82.3] days).
Conclusions and relevance: In this study, approximately half of pregnant women discontinued SSRIs or SNRIs, and discontinuation during pregnancy was not associated with adverse psychiatric-related outcomes, including hospitalizations, outpatient visits, suicidal behavior, or sick leave absence in the 90 days or 1.5 years after childbirth.
Conflict of interest statement
Figures

References
-
- Molenaar NM, Bais B, Lambregtse-van den Berg MP, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J Affect Disord. 2020;264:82-89. doi: 10.1016/j.jad.2019.12.014 - DOI - PubMed
-
- Liu X, Musliner KL, Agerbo E, et al. Treatment indications for antidepressants prescribed to pregnant women: a population-based descriptive study from Denmark. Pharmacoepidemiol Drug Saf. 2020;29(3):347-351. doi: 10.1002/pds.4953 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

