HCN1 hyperpolarization-activated cyclic nucleotide-gated channels enhance evoked GABA release from parvalbumin-positive interneurons
- PMID: 39378096
- PMCID: PMC11494348
- DOI: 10.1073/pnas.2319246121
HCN1 hyperpolarization-activated cyclic nucleotide-gated channels enhance evoked GABA release from parvalbumin-positive interneurons
Abstract
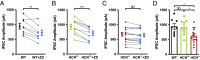
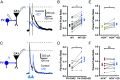
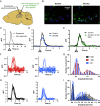
Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels generate the cationic Ih current in neurons and regulate the excitability of neuronal networks. The function of HCN channels depends, in part, on their subcellular localization. Of the four HCN isoforms (HCN1-4), HCN1 is strongly expressed in the dendrites of pyramidal neurons (PNs) in hippocampal area CA1 but also in presynaptic terminals of parvalbumin-positive interneurons (PV+ INs), which provide strong inhibitory control over hippocampal activity. Yet, little is known about how HCN1 channels in these cells regulate the evoked release of the inhibitory transmitter GABA from their axon terminals. Here, we used genetic, optogenetic, electrophysiological, and imaging techniques to investigate how the electrophysiological properties of PV+ INs are regulated by HCN1, including how HCN1 activity at presynaptic terminals regulates the release of GABA onto PNs in CA1. We found that application of HCN1 pharmacological blockers reduced the amplitude of the inhibitory postsynaptic potential recorded from CA1 PNs in response to selective optogenetic stimulation of PV+ INs. Homozygous HCN1 knockout mice also show reduced IPSCs in postsynaptic cells. Finally, two-photon imaging using genetically encoded fluorescent calcium indicators revealed that HCN1 blockers reduced the probability that an extracellular electrical stimulating pulse evoked a Ca2+ response in individual PV+ IN presynaptic boutons. Taken together, our results show that HCN1 channels in the axon terminals of PV+ interneurons facilitate GABAergic transmission in the hippocampal CA1 region.
Keywords: HCN channel; hippocampus; interneuron; parvalbumin; synapse.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

