Octreotide Subcutaneous Depot for Acromegaly: A Randomized, Double-blind, Placebo-controlled Phase 3 Trial, ACROINNOVA 1
- PMID: 39378125
- PMCID: PMC12086390
- DOI: 10.1210/clinem/dgae707
Octreotide Subcutaneous Depot for Acromegaly: A Randomized, Double-blind, Placebo-controlled Phase 3 Trial, ACROINNOVA 1
Abstract
Context: Acromegaly, characterized by excessive GH and insulin-like growth factor-1 (IGF-1), impacts quality of life (QoL) and mortality. Standard of care (SoC; octreotide long-acting repeatable or lanreotide autogel) treatment typically requires healthcare provider administration. CAM2029, a novel subcutaneous octreotide depot with increased bioavailability using FluidCrystal technology, enables self-administration and room-temperature storage.
Objective: Assess superiority of CAM2029 vs placebo for biochemical control in patients with controlled acromegaly.
Design: 24-week, multinational, randomized, double-blind, phase 3 trial (NCT04076462).
Setting: 45 sites; 10 countries.
Patients: 72 patients on SoC with biochemical control at screening [IGF-1 ≤upper limit of normal (ULN); mean GH <2.5 μg/L].
Interventions: Patients were randomized 2:1 to once-monthly CAM2029 (n = 48) or placebo (n = 24).
Main outcome measures: The primary endpoint was proportion of patients with IGF-1 ≤ULN (week 22/24 mean), with dose-reduced patients classified as nonresponders; first key secondary endpoint was the same, including dose-reduced responders. The second key secondary endpoint was proportion of patients with IGF-1 ≤ULN (week 22/24) and mean GH <2.5 μg/L (week 24).
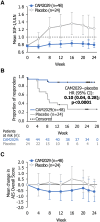
Results: At week 22/24 (intention-to-treat analysis), CAM2029-treated patients demonstrated superior response rates vs placebo for IGF-1 (72.2% vs 37.5%; risk difference: 34.6, 95% confidence interval: 11.3, 57.9; P = .0018) and combined IGF-1/GH (70.0% vs 37.5%; P = .0035). CAM2029-treated patients had well-controlled symptoms, improved QoL, and treatment satisfaction vs placebo and baseline. CAM2029 was well tolerated; safety was consistent with SoC.
Conclusion: CAM2029 provides a convenient and effective treatment option for acromegaly, with superior biochemical control vs placebo. Symptom control, QoL, and satisfaction were improved from baseline SoC.
Clinical trial registration: NCT04076462 (ClinicalTrials.gov).
Keywords: CAM2029; FluidCrystal; acromegaly; octreotide; randomized controlled trial; somatostatin receptor ligands.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Crisafulli S, Luxi N, Sultana J, et al. Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur J Endocrinol. 2021;185(2):251‐263. - PubMed
-
- Katznelson L, Laws ERJr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933‐3951. - PubMed
-
- Colao A, Grasso LFS, Giustina A, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

