A modified BPaL regimen for tuberculosis treatment replaces linezolid with inhaled spectinamides
- PMID: 39378165
- PMCID: PMC11460978
- DOI: 10.7554/eLife.96190
A modified BPaL regimen for tuberculosis treatment replaces linezolid with inhaled spectinamides
Abstract
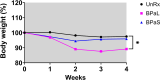
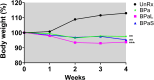
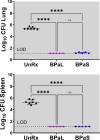
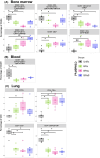
The Nix-TB clinical trial evaluated a new 6 month regimen containing three oral drugs; bedaquiline (B), pretomanid (Pa), and linezolid (L) (BPaL regimen) for the treatment of tuberculosis (TB). This regimen achieved remarkable results as almost 90% of the multidrug-resistant or extensively drug-resistant TB participants were cured but many patients also developed severe adverse events (AEs). The AEs were associated with the long-term administration of the protein synthesis inhibitor linezolid. Spectinamide 1599 is also a protein synthesis inhibitor of Mycobacterium tuberculosis with an excellent safety profile, but it lacks oral bioavailability. Here, we propose to replace L in the BPaL regimen with spectinamide (S) administered via inhalation and we demonstrate that inhaled spectinamide 1599, combined with BPa --BPaS regimen--has similar efficacy to that of the BPaL regimen while simultaneously avoiding the L-associated AEs. The BPaL and BPaS regimens were compared in the BALB/c and C3HeB/FeJ murine chronic TB efficacy models. After 4-weeks of treatment, both regimens promoted equivalent bactericidal effects in both TB murine models. However, treatment with BPaL resulted in significant weight loss and the complete blood count suggested the development of anemia. These effects were not similarly observed in mice treated with BPaS. BPaL and BPa, but not the BPaS treatment, also decreased myeloid to erythroid ratio suggesting the S in the BPaS regimen was able to recover this effect. Moreover, the BPaL also increased concentration of proinflammatory cytokines in bone marrow compared to mice receiving BPaS regimen. These combined data suggest that inhaled spectinamide 1599 combined with BPa is an effective TB regimen without L-associated AEs.
Keywords: Mycobacterium; bedaquiline/pretomanid; infectious disease; inhalation; linezolid; microbiology; spectinmaides; tuberculosis.
© 2024, Zohaib Ali et al.
Conflict of interest statement
MZ, TD, AM, AW, CP, HL, JP, JP, MH, RL, JL, GR, AH, BM, MG No competing interests declared
Figures

















Update of
-
A Modified BPaL Regimen for Tuberculosis Treatment replaces Linezolid with Inhaled Spectinamides.bioRxiv [Preprint]. 2024 Jun 11:2023.11.16.567434. doi: 10.1101/2023.11.16.567434. bioRxiv. 2024. Update in: Elife. 2024 Oct 08;13:RP96190. doi: 10.7554/eLife.96190. PMID: 38014249 Free PMC article. Updated. Preprint.
References
-
- Bigelow KM, Tasneen R, Chang YS, Dooley KE, Nuermberger EL. Preserved efficacy and reduced toxicity with intermittent linezolid dosing in combination with bedaquiline and pretomanid in a murine tuberculosis model. Antimicrobial Agents and Chemotherapy. 2020;64:e01178-20. doi: 10.1128/AAC.01178-20. - DOI - PMC - PubMed
-
- Bigelow KM, Deitchman AN, Li SY, Barnes-Boyle K, Tyagi S, Soni H, Dooley KE, Savic RM, Nuermberger EL. Pharmacodynamic correlates of linezolid activity and toxicity in murine models of tuberculosis. The Journal of Infectious Diseases. 2021;223:1855–1864. doi: 10.1093/infdis/jiaa016. - DOI - PMC - PubMed
-
- Boisson M, Jacobs M, Grégoire N, Gobin P, Marchand S, Couet W, Mimoz O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrobial Agents and Chemotherapy. 2014;58:7331–7339. doi: 10.1128/AAC.03510-14. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

