Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain
- PMID: 39378238
- PMCID: PMC11465073
- DOI: 10.1085/jgp.202413596
Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain
Abstract
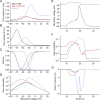
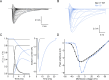
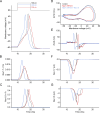
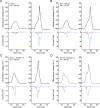
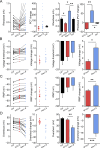
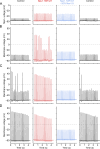
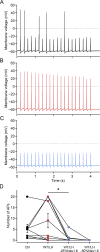
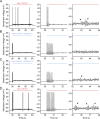
While voltage-gated sodium channels Nav1.7 and Nav1.8 both contribute to electrogenesis in dorsal root ganglion (DRG) neurons, details of their interactions have remained unexplored. Here, we studied the functional contribution of Nav1.8 in DRG neurons using a dynamic clamp to express Nav1.7L848H, a gain-of-function Nav1.7 mutation that causes inherited erythromelalgia (IEM), a human genetic model of neuropathic pain, and demonstrate a profound functional interaction of Nav1.8 with Nav1.7 close to the threshold for AP generation. At the voltage threshold of -21.9 mV, we observed that Nav1.8 channel open-probability exceeded Nav1.7WT channel open-probability ninefold. Using a kinetic model of Nav1.8, we showed that a reduction of Nav1.8 current by even 25-50% increases rheobase and reduces firing probability in small DRG neurons expressing Nav1.7L848H. Nav1.8 subtraction also reduces the amplitudes of subthreshold membrane potential oscillations in these cells. Our results show that within DRG neurons that express peripheral sodium channel Nav1.7, the Nav1.8 channel amplifies excitability at a broad range of membrane voltages with a predominant effect close to the AP voltage threshold, while Nav1.7 plays a major role at voltages closer to resting membrane potential. Our data show that dynamic-clamp reduction of Nav1.8 conductance by 25-50% can reverse hyperexcitability of DRG neurons expressing a gain-of-function Nav1.7 mutation that causes pain in humans and suggests, more generally, that full inhibition of Nav1.8 may not be required for relief of pain due to DRG neuron hyperexcitability.
© 2024 Vasylyev et al.
Conflict of interest statement
Disclosures: S.G. Waxman reported personal fees from Amgen, personal fees from Biogen, personal fees from Glaxo, personal fees from Eli Lilly, personal fees from ThirdRock Ventures, personal fees from Olipass Biotherapeutics, personal fees from Sangamo Therapeutics, personal fees from Exicure, personal fees from Chromocell Therapeutics, personal fees from Latigo Labs, personal fees from Envida, personal fees from Ionis, personal fees from Almylan, personal fees from Forestie Labs, personal fees from Argo Therapeutics, personal fees from Neurona, personal fees from Shape Therapeutics, personal fees from NxGen, personal fees from Arrowhead Therapeutics, personal fees from Voyager Therapeutics, other from Site One Therapeutics, other from Navega Therapeutics, personal fees from Medtronic, and other from Vertex Therapeutics outside the submitted work. No other disclosures were reported.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

