Estimating Long-Term Survivorship Rates Among Patients With Resected Stage III/IV Melanoma: Analyses From CheckMate 238 and European Organization for Research and Treatment of Cancer 18071 Trials
- PMID: 39378385
- PMCID: PMC11895827
- DOI: 10.1200/JCO.24.00237
Estimating Long-Term Survivorship Rates Among Patients With Resected Stage III/IV Melanoma: Analyses From CheckMate 238 and European Organization for Research and Treatment of Cancer 18071 Trials
Abstract
Purpose: Standard-of-care treatments for patients with resected stage III/IV melanoma include the immuno-oncology (IO) agents nivolumab (NIVO) and ipilimumab (IPI). This study used mixture cure models (MCMs) to estimate cure rates among patients treated with NIVO or IPI in the phase III CheckMate 238 (ClinicalTrials.gov identifier: NCT02388906) and European Organization for Research and Treatment of Cancer (EORTC) 18071 (ClinicalTrials.gov identifier: NCT00636168) trials, and to assess the impact of use of adjuvant immunotherapy on cure rates versus watchful waiting.
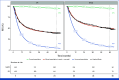
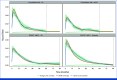
Methods: MCMs were applied to patient-level recurrence-free survival data from CheckMate 238 and EORTC 18071. Cured patients were assumed to experience no disease recurrence and mortality risks similar to the general population. Uncured patients were at risk of disease recurrence and all-cause death. The survival trend of the cured patients was estimated using life expectancy data for a general population with the same baseline demographic characteristics. A regression model assessed the odds ratios (ORs) of cure across key subgroups on the basis of baseline characteristics of the study populations.
Results: In CheckMate 238, estimated cure rates were 48.3% (95% CI, 41.8 to 54.9) with NIVO and 38.2% (95% CI, 32.7 to 44.1) with IPI. In EORTC 18071, estimated cure rates were 38.0% (95% CI, 32.1 to 44.2) with IPI and 29.2% (95% CI, 24.4 to 34.6) with placebo. In the indirect comparison of the two trials, the odds of cure were significantly higher with NIVO than with placebo (OR, 2.33 [95% CI, 1.49 to 3.65]).
Conclusion: Analyses involving two large phase III trials investigating adjuvant IO treatment for resected melanoma demonstrate higher cure rates for both NIVO and IPI than placebo, with NIVO providing the highest cure rate. Similar cure rates were estimated for patients treated with IPI in both trials, despite staging and dosing differences.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures




References
-
- Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur J Cancer. 2020;126:141–158. - PubMed
-
- Joyce KM. Surgical management of melanoma Ward WH, Farma JM. Cutaneous Melanoma: Etiology and Therapy Brisbane, AU: Codon Publications; 2017, pp 91-100 - PubMed
-
- Leiter U, Buettner PG, Eigentler TK, et al. Prognostic factors of thin cutaneous melanoma: An analysis of the central malignant melanoma registry of the German Dermatological Society. J Clin Oncol. 2004;22:3660–3667. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

