NSGS mice humanized with cord blood mononuclear cells show sustained and functional myeloid-lymphoid representation with limited graft-versus-host disease
- PMID: 39379097
- PMCID: PMC11459296
- DOI: 10.1136/jitc-2024-009198
NSGS mice humanized with cord blood mononuclear cells show sustained and functional myeloid-lymphoid representation with limited graft-versus-host disease
Abstract
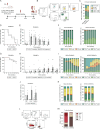
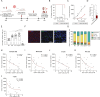
Humanized immunodeficient mice serve as critical models for investigating the functional interplay between transplanted human cells and a pre-reconstituted human immune system. These models facilitate the study of molecular and cellular pathogenic mechanisms and enable the evaluation of the efficacy and toxicity of immunotherapies, thereby accelerating their preclinical and clinical development. Current strategies rely on inefficient, long-term/delayed hematopoietic reconstitution by CD34+ hematopoietic progenitors or short-term reconstitution with peripheral blood mononuclear cells (PB-MNCs) associated with high rates of graft-versus-host disease (GvHD) and an inefficient representation of immune cell populations. Here, we hypothesized that immunologically naïve cord blood mononuclear cells (CB-MNCs) could serve as a superior alternative, providing long-lasting and functionally effective immune reconstitution. We conducted a comprehensive comparison between the non-obese diabetic (NOD).Cg-Prkdc∧ˆscid-IL2rg∧ˆtm1Wjl/SzJ (NSG) and NSG-Tg(CMV-IL3,CSF2,KITLG)∧ˆ1Eav/MloySzJ (NSGS) immunodeficient mouse models following humanization with either PB-MNCs or CB-MNCs. We assessed the engraftment dynamics of various human immune cells over time and monitored the development of GvHD in both models. For the most promising model, we extensively evaluated immune cell functionality in vitro and in vivo using sarcoma and leukemia xenografts. Humanizing NSGS mice with CB-MNCs results in a rapid, robust, and sustained representation of a diverse range of functional human lymphoid and myeloid cell populations while minimizing GvHD incidence. In this model, human immune cell populations significantly impair the growth and engraftment of sarcoma and B-cell acute lymphoblastic leukemia cells, with a significant inverse correlation between immune cell levels and tumor growth. This study establishes a fast, efficient, and reliable in vivo platform for various applications in cancer immunotherapy, particularly for exploring the complex interactions between cancer cells, immune cells, and the tumor microenvironment in vivo, prior to clinical development.
Keywords: Engraftment; Graft versus leukemia; Immunotherapy; Solid tumor; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PM is founder of the spin-off OneChain Immunotherapeutics, which has no connection with the present research. The remaining authors declare no competing interests.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
