EASIX-guided risk stratification for complications and outcome after CAR T-cell therapy with ide-cel in relapsed/refractory multiple myeloma
- PMID: 39379098
- PMCID: PMC11459298
- DOI: 10.1136/jitc-2024-009220
EASIX-guided risk stratification for complications and outcome after CAR T-cell therapy with ide-cel in relapsed/refractory multiple myeloma
Abstract
Background: Chimeric antigen receptor (CAR) T-cell therapy has demonstrated significant benefits in the treatment of relapsed/refractory multiple myeloma (RRMM). However, these outcomes can be compromised by severe complications, including cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome (ICANS) and immune effector cell-associated hematotoxicity (ICAHT), predisposing for life-threatening infections.
Methods: This retrospective observational study examined a total of 129 patients with RRMM who had received idecabtagene vicleucel (ide-cel) at two major myeloma centers in Germany and one center in the USA to assess the Endothelial Activation and Stress Index (EASIX) as a risk marker for an unfavorable clinical course and outcome after CAR T-cell therapy. EASIX is calculated by lactate dehydrogenase (U/L) × creatinine (mg/dL) / platelets (109 cells/L) and was determined before lymphodepletion (baseline) and at the day of CAR T-cell infusion (day 0). The analysis was extended to EASIX derivatives and the CAR-HEMATOTOX score.
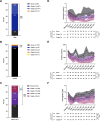
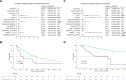
Results: An elevated baseline EASIX (>median) was identified as a risk marker for severe late ICAHT, manifesting with an impaired hematopoietic reconstitution and pronounced cytopenias during the late post-CAR-T period. Patients with high EASIX levels (>upper quartile) were particularly at risk, as evidenced by an increased rate of an aplastic phenotype of neutrophil recovery, severe late-onset infections and ICANS. Finally, we found associations between baseline EASIX and an inferior progression-free and overall survival. Moreover, the EASIX at day 0 also demonstrated potential to serve as a risk marker for post-CAR-T complications and adverse outcomes.
Conclusions: In conclusion, EASIX aids in risk stratification at clinically relevant time points prior to CAR T-cell therapy with ide-cel. Increased EASIX levels might help clinicians to identify vulnerable patients to adapt peri-CAR-T management at an early stage.
Keywords: chimeric antigen receptor - CAR; cytopenia; immunotherapy; multiple myeloma; treatment related adverse event - trAE.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JF has received honoraria from BMS and Stemline and travel and congress participation grants from Janssen-Cilag. XZ declares advisory services for and has received travel support from SkylineDx. JK has received honoraria from AstraZeneca. EKM declares a consulting or advisory role for Amgen, BMS/Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Sanofi, Stemline and Takeda. He has received honoraria from Amgen, BMS/Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Sanofi, Stemline and Takeda, research funding from BMS/Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi and Takeda and travel support from BMS/Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi, Stemline and Takeda. MJF declares consulting activity for Pfizer and Kerna Ventures. CSM has received honoraria and travel support from Janssen-Cilag. IB has received honoraria from Oncopeptide and travel support from Janssen. ON reports receiving consulting fees from Janssen, BMS, Takeda, GPCR therapeutics, Sanofi and Pfizer. AS has received travel grants from Hexal and Jazz Pharmaceuticals and research grants from Therakos/Mallinckrodt. She is a consultant for Janssen-Cilag and BMS and co-founder of TolerogenixX Ltd. AS is part-time employee of TolerogenixX Ltd. MS declares an advisory role or expert testimony for MSD, Novartis, BMS and Pierre Fabre. He is co-founder and shareholder of TolerogenixX GmbH, Heidelberg. He has received financial support for research on biosimilars and travel grants from Hexal, financial support of educational activities and conference participation and travels grants from Kite and BMS, collaborative research grants from Novartis and funding for collaborative research from Apogenix. MT has received research funding from Kite, Regeneron and Roche. He is an advisory board member for AstraZeneca, BMS, Incyte, Janssen and Novartis. HE declares a consulting or advisory role for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, Novartis and Roche. He has received research funding from BMS/Celgene, Janssen, Amgen, GSK, Sanofi and Novartis, honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, Novartis and travel support from BMS/Celgene, Janssen and Amgen. PD reports consultancy for AbbVie, AstraZeneca, Beigene, BMS, Gilead, Miltenyi, Novartis and Riemser. He is member of the speakers’ bureau for AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Novartis, Riemser and Roche and has received research support from Riemser (all to institution). NCM reports receiving personal fees from BMS, Janssen, Amgen, Takeda, OncoPep, AbbVie, Karyopharm, Novartis, Legend, Raqia, Adaptive Biotechnology, and Pfizer outside the submitted work. He has intellectual property licensed to OncoPep and held stocks in C4 Therapeutics. ASp reports receiving consulting fees from Novartis and Roche. LR consulted for Janssen, Amgen, GSK, Pfizer, BMS, Sanofi, and received honoraria from Janssen, GSK, Pfizer, BMS, Sanofi and received research funding from Skyline Dx and BMS. SS has received travel grants or honoraria for presentations from Celgene, BMS, Janssen, Takeda and Amgen. MSR declares a consulting or advisory role for BMS, Amgen, GSK, Janssen, Sanofi, Pfizer, AbbVie and Takeda. He has received research funding from BMS, Janssen, Sanofi and Heidelberg Pharma, travel support from BMS, Amgen and Janssen and honoraria from BMS, Janssen, AbbVie and Sanofi.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
