Acid-Sensing Ion Channels Drive the Generation of Tactile Impulses in Merkel Cell-Neurite Complexes of the Glabrous Skin of Rodent Hindpaws
- PMID: 39379156
- PMCID: PMC11580779
- DOI: 10.1523/JNEUROSCI.0885-24.2024
Acid-Sensing Ion Channels Drive the Generation of Tactile Impulses in Merkel Cell-Neurite Complexes of the Glabrous Skin of Rodent Hindpaws
Abstract
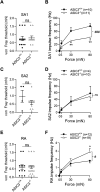
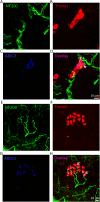
Merkel cell-neurite complexes (MNCs) are enriched in touch-sensitive areas, including whisker hair follicles and the glabrous skin of the rodent's paws, where tactile stimulation elicits slowly adapting type 1 (SA1) tactile impulses to encode for the sense of touch. Recently, we have shown with rodent whisker hair follicles that SA1 impulses are generated through fast excitatory synaptic transmission at MNCs and driven by acid-sensing ion channels (ASICs). However, it is currently unknown whether, besides whisker hair follicles, ASICs also play an essential role in generating SA1 impulses from MNCs of other body parts in mammals. In the present study, we attempted to address this question by using the skin-nerve preparations made from the hindpaw glabrous skin and tibial nerves of both male and female rodents and applying the pressure-clamped single-fiber recordings. We showed that SA1 impulses elicited by tactile stimulation to the rat hindpaw glabrous skin were largely diminished in the presence of amiloride and diminazene, two ASIC channel blockers. Furthermore, using the hindpaw glabrous skin and tibial nerve preparations made from the mice genetically deleted of ASIC3 channels (ASIC3-/-), we showed that the frequency of SA1 impulses was significantly lower in ASIC3-/- mice than in littermate wild-type ASIC3+/+ mice, a result consistent with the pharmacological experiments with ASIC channel blockers. Our findings suggest that ASIC channels are essential for generating SA1 impulses to underlie the sense of touch in the glabrous skin of rodent hindpaws.
Keywords: Merkel cell–neurite complex; Piezo2 channel; acid-sensing ion channel; low threshold mechanoreceptor; sense of touch; slowly adapting type 1 response.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
