Metabolic regulation of the immune system in health and diseases: mechanisms and interventions
- PMID: 39379377
- PMCID: PMC11461632
- DOI: 10.1038/s41392-024-01954-6
Metabolic regulation of the immune system in health and diseases: mechanisms and interventions
Abstract
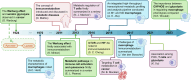
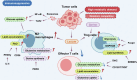
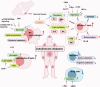
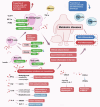
Metabolism, including glycolysis, oxidative phosphorylation, fatty acid oxidation, and other metabolic pathways, impacts the phenotypes and functions of immune cells. The metabolic regulation of the immune system is important in the pathogenesis and progression of numerous diseases, such as cancers, autoimmune diseases and metabolic diseases. The concept of immunometabolism was introduced over a decade ago to elucidate the intricate interplay between metabolism and immunity. The definition of immunometabolism has expanded from chronic low-grade inflammation in metabolic diseases to metabolic reprogramming of immune cells in various diseases. With immunometabolism being proposed and developed, the metabolic regulation of the immune system can be gradually summarized and becomes more and more clearer. In the context of many diseases including cancer, autoimmune diseases, metabolic diseases, and many other disease, metabolic reprogramming occurs in immune cells inducing proinflammatory or anti-inflammatory effects. The phenotypic and functional changes of immune cells caused by metabolic regulation further affect and development of diseases. Based on experimental results, targeting cellular metabolism of immune cells becomes a promising therapy. In this review, we focus on immune cells to introduce their metabolic pathways and metabolic reprogramming, and summarize how these metabolic pathways affect immune effects in the context of diseases. We thoroughly explore targets and treatments based on immunometabolism in existing studies. The challenges of translating experimental results into clinical applications in the field of immunometabolism are also summarized. We believe that a better understanding of immune regulation in health and diseases will improve the management of most diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Novel lncRNA LncMSTRG.11341.25 Promotes Osteogenic Differentiation of Human Bone Marrow Stem Cells via the miR-939-5p/PAX8 Axis.Research (Wash D C). 2025 Feb 6;8:0601. doi: 10.34133/research.0601. eCollection 2025. Research (Wash D C). 2025. PMID: 39916798 Free PMC article.
-
Recent advances in self-targeting natural product-based nanomedicines.J Nanobiotechnology. 2025 Jan 20;23(1):31. doi: 10.1186/s12951-025-03092-9. J Nanobiotechnology. 2025. PMID: 39833846 Free PMC article. Review.
-
Unraveling the triad of immunotherapy, tumor microenvironment, and skeletal muscle biomechanics in oncology.Front Immunol. 2025 Apr 2;16:1572821. doi: 10.3389/fimmu.2025.1572821. eCollection 2025. Front Immunol. 2025. PMID: 40242775 Free PMC article. Review.
-
The immune tolerance role of Bregs in inhibiting human inflammatory diseases, with a focus on diabetes mellitus.Front Immunol. 2025 Apr 30;16:1565158. doi: 10.3389/fimmu.2025.1565158. eCollection 2025. Front Immunol. 2025. PMID: 40370441 Free PMC article. Review.
-
Anti-DAMP therapies for acute inflammation.Front Immunol. 2025 May 8;16:1579954. doi: 10.3389/fimmu.2025.1579954. eCollection 2025. Front Immunol. 2025. PMID: 40406124 Free PMC article. Review.
References
-
- Fullerton, M. D., Steinberg, G. R. & Schertzer, J. D. Immunometabolism of AMPK in insulin resistance and atherosclerosis. Mol. Cell Endocrinol.366, 224–234 (2013). - PubMed
-
- Schipper, H. S., Prakken, B., Kalkhoven, E. & Boes, M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab.23, 407–415 (2012). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

