Presence of cholestasis and its impact on survival in SARS-CoV-2 associated acute respiratory distress syndrome
- PMID: 39379494
- PMCID: PMC11461911
- DOI: 10.1038/s41598-024-73948-x
Presence of cholestasis and its impact on survival in SARS-CoV-2 associated acute respiratory distress syndrome
Abstract
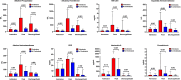
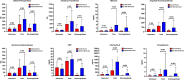
Data on cholestasis and biliary injury in patients with COVID-19 are scarce. The primary aim of this study was to evaluate the prevalence of cholestasis and factors associated with its development and outcome in critically ill patients with COVID-19 associated acute respiratory distress syndrome (ARDS). In this retrospective exploratory study, COVID-19 patients with ARDS admitted to an intensive care unit (ICU) at the Medical University of Vienna were evaluated for the development of cholestasis defined as an alkaline phosphatase level of 1.67x upper limit of normal for at least three consecutive days. Simple and multiple logistic regression analysis was used to evaluate parameters associated with development of cholestasis and survival. Of 225 included patients 119 (53%) developed cholestasis during ICU stay. Patients with cholestasis had higher peak levels of alkaline phosphatase, gamma-glutamyl transferase, bilirubin and inflammation parameters. Factors independently associated with cholestasis were extracorporeal membrane oxygenation support, ketamine use, high levels of inflammation parameters and disease severity. Presence of cholestasis and peak ALP levels were independently associated with worse ICU and 6-month survival. Development of cholestasis is a common complication in critically ill COVID-19 patients and represents a negative prognostic marker for survival. It is associated with disease severity and specific treatment modalities of intensive care.
Keywords: COVID-19; Cholestasis; ICU; Ketamine; Liver injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
M.T. received speaker fees from BMS, Falk Foundation, Gilead, Intercept, Ipsen, Jannsen, Madrigal, MSD and Roche; he advised for Abbvie, Agomab, Albireo, BiomX, Boehringer Ingelheim, Chemomab, Cymabay, Falk Pharma GmbH, Genfit, Gilead, Hightide, Intercept, Janssen, LoopLab, Mirum, MSD, Novartis, Phenex, Pliant, Rectify, Regulus, Siemens and Shire. He further received travel support from Abbvie, Falk, Gilead, Intercept and Jannsen and research grants from Albireo, Alnylam, Cymabay, Falk, Genentech, Gilead, Intercept, MSD, Takeda and UltraGenyx. He is also co-inventor of patents on the medical use of norUDCA filed by the Medical Universities of Graz and Vienna. K.K. received research grants from Apeptico, Biotest, Bayer and Alterras and travel support from Biotest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

