Role of 18F-FDG-PET/CT in the initial staging of very high-risk Ewing Sarcoma in a prospective multicentric Phase II Study: Is there still a place for bone marrow sampling?
- PMID: 39379569
- PMCID: PMC11554785
- DOI: 10.1038/s41416-024-02864-8
Role of 18F-FDG-PET/CT in the initial staging of very high-risk Ewing Sarcoma in a prospective multicentric Phase II Study: Is there still a place for bone marrow sampling?
Abstract
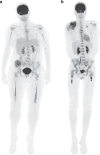
Background: The Ewing Sarcoma Family of Tumors (ESFT) constitutes a group of rare malignancies, wherein approximately one-third of cases exhibit metastatic spread, particularly impacting prognosis when bone and/or bone marrow (BM) are involved. Primary extra-pulmonary metastatic ESFT often necessitates intensified therapeutic approaches. Accurate staging plays a pivotal role in clinical decision-making, with fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (PET/CT) currently serving as a non-invasive modality for assessing ESFT's BM extent.
Methods: In the French phase II COMBINAIR3 (NCT03011528) study, a comprehensive approach for patients with extra-pulmonary ESFT metastasis was evaluated. We prospectively compared the efficacy of PET/CT to BM aspiration and biopsy (BMAB) analysis in patients undergoing initial staging.
Results: Among the 42 patients analyzed (median age 14 y, 2:1 male/female ratio), 45% presented with pelvic primary tumors and 83% had bone/BM involvement at diagnosis. Our findings showed PET/CT had 100% specificity and 83.3% sensitivity in detecting initial BM involvement. Overall, PET/CT correctly classified 92.8% of patients, reaching 100% accuracy in patients identified with bone involvement, thus surpassing the standard BMAB.
Discussion: These results suggest that the conventional use of BMAB in the initial staging of high-risk ESFT patients can be omitted, promoting PET/CT as a non-invasive alternative, thus improving staging accuracy and treatment decisions in ESFT management.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures




References
-
- Fletcher CDM, World Health Organization, International Agency for Research on Cancer, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. 468 (World Health Organization classification of tumours).
-
- Dockhorn-Dworniczak B, Schäfer KL, Dantcheva R, Blasius S, Winkelmann W, Strehl S, et al. Diagnostic value of the molecular genetic detection of the t(11;22) translocation in Ewing’s tumours. Virchows Arch Int J Pathol. 1994;425:107–12. - PubMed
-
- Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumors–a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N. Engl J Med. 1994;331(Aug 4):294–9. - PubMed
-
- Kovar H, Dworzak M, Strehl S, Schnell E, Ambros IM, Ambros PF, et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. Oncogene. 1990;5(Jul):1067–70. - PubMed
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

