Silencing of SIRPα enhances the antitumor efficacy of CAR-M in solid tumors
- PMID: 39379603
- PMCID: PMC11527885
- DOI: 10.1038/s41423-024-01220-3
Silencing of SIRPα enhances the antitumor efficacy of CAR-M in solid tumors
Abstract
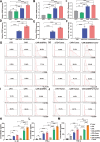
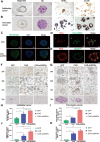
The potential of macrophage-mediated phagocytosis as a cancer treatment is promising. Blocking the CD47-SIRPα interaction with a CD47-specific antibody significantly enhances macrophage phagocytosis. However, concerns regarding their toxicity to nontumor cells remain substantial. Here, we engineered chimeric antigen receptor macrophages (CAR-Ms) by fusing a humanized single-chain variable fragment with FcγRIIa and integrating short hairpin RNA to silence SIRPα, thereby disrupting the CD47-SIRPα signaling pathway. These modified CAR-shSIRPα-M cells exhibited an M1-like phenotype, superior phagocytic function, substantial cytotoxic effects on HER2-positive tumor cells, and the ability to eliminate patient-derived organoids. In vivo, CAR-M cells significantly inhibited tumor growth and prolonged survival in tumor-bearing mice. Notably, CAR-shSIRPα-M cells enhanced cytotoxic T-cell infiltration into tumors, thereby enhancing the antitumor response in both the humanized immune system mouse model and immunocompetent mice. Mechanistically, SIRPα inhibition activated inflammatory pathways and the cGAS-STING signaling cascade in CAR-M cells, leading to increased production of proinflammatory cytokines, reactive oxygen species, and nitric oxide, thereby enhancing their antitumor effects. These findings underscore the potential of SIRPα inhibition as a novel strategy to increase the antitumor efficacy of CAR-M cells in cancer immunotherapy, particularly against solid tumors.
Keywords: CAR-M; Cancer immunotherapy; Phagocytosis; SIRPα; Solid tumor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

