Type 17 immunity: novel insights into intestinal homeostasis and autoimmune pathogenesis driven by gut-primed T cells
- PMID: 39379604
- PMCID: PMC11528014
- DOI: 10.1038/s41423-024-01218-x
Type 17 immunity: novel insights into intestinal homeostasis and autoimmune pathogenesis driven by gut-primed T cells
Abstract
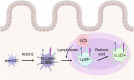
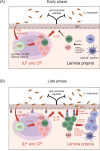
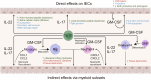
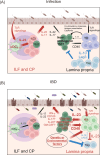
The IL-23 signaling pathway in both innate and adaptive immune cells is vital for orchestrating type 17 immunity, which is marked by the secretion of signature cytokines such as IL-17, IL-22, and GM-CSF. These proinflammatory mediators play indispensable roles in maintaining intestinal immune equilibrium and mucosal host defense; however, their involvement has also been implicated in the pathogenesis of chronic inflammatory disorders, such as inflammatory bowel diseases and autoimmunity. However, the implications of type 17 immunity across diverse inflammation models are complex. This review provides a comprehensive overview of the multifaceted roles of these cytokines in maintaining gut homeostasis and in perturbing gut barrier integrity, leading to acute and chronic inflammation in various models of gut infection and colitis. Additionally, this review focuses on type 17 immunity interconnecting multiple organs in autoimmune conditions, with a particular emphasis on the pathogenesis of autoimmune arthritis and neuroinflammation driven by T cells primed within the gut microenvironment.
Keywords: Gut-brain axis; Gut-joint axis; IL-23; Inflammatory bowel diseases (IBD); Th17; cDCIL-23.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. 10.1016/S1074-7613(00)00070-4. - PubMed
-
- Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41:283–97. 10.1007/S00281-019-00733-8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

