Heparin treatment is associated with a delayed diagnosis of Alzheimer's dementia in electronic health records from two large United States health systems
- PMID: 39379683
- PMCID: PMC11919696
- DOI: 10.1038/s41380-024-02757-5
Heparin treatment is associated with a delayed diagnosis of Alzheimer's dementia in electronic health records from two large United States health systems
Abstract
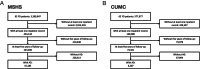
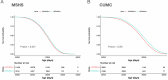
Recent studies suggest that heparan sulfate proteoglycans (HSPG) contribute to the predisposition to, protection from, and potential treatment and prevention of Alzheimer's disease (AD). Here, we used electronic health records (EHR) from two different health systems to examine whether heparin therapy was associated with a delayed diagnosis of AD dementia. Longitudinal EHR data from 15,183 patients from the Mount Sinai Health System (MSHS) and 6207 patients from Columbia University Medical Center (CUMC) were used in separate survival analyses to compare those who did or did not receive heparin therapy, had a least 5 years of observation, were at least 65 years old by their last visit, and had subsequent diagnostic code or drug treatment evidence of possible AD dementia. Analyses controlled for age, sex, comorbidities, follow-up duration and number of inpatient visits. Heparin therapy was associated with significant delays in age of clinical diagnosis of AD dementia, including +1.0 years in the MSMS cohort (P < 0.001) and +1.0 years in the CUMC cohort (P < 0.001). While additional studies are needed, this study supports the potential roles of heparin-like drugs and HSPGs in the protection from and prevention of AD dementia.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: YTQ reports consulting fees from Biogen. YTQ, JFAV, EMR are listed as co-inventors of a patent application for therapeutics modulating interactions between APOE and HSPG filed by MGB. Ethics approval and consent to participate: This retrospective cohort study received Institutional Review Board (IRB) approval from all participating institutions (MSHS: 19-00951, CUMC: AAAL0601). In both cases, the IRB committee waived informed consent.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

