Efficacy and Safety of Andexanet Alfa Versus Four Factor Prothrombin Complex Concentrate for Emergent Reversal of Factor Xa Inhibitor Associated Intracranial Hemorrhage: A Systematic Review and Meta-Analysis
- PMID: 39379749
- PMCID: PMC11950062
- DOI: 10.1007/s12028-024-02130-y
Efficacy and Safety of Andexanet Alfa Versus Four Factor Prothrombin Complex Concentrate for Emergent Reversal of Factor Xa Inhibitor Associated Intracranial Hemorrhage: A Systematic Review and Meta-Analysis
Abstract
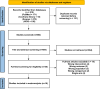
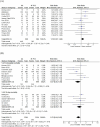
Factor Xa inhibitors (FXaI) are increasingly used for anticoagulation therapy, yet their association with intracranial hemorrhage poses a significant challenge. Although andexanet alfa (AA) and four-factor prothrombin complex concentrate (4F-PCC) have shown promise in reversing FXaI effects, their comparative efficacy and safety remain uncertain. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we conducted a literature search on electronic databases to obtain the relevant studies until May 16, 2024. Our primary outcomes were successful anticoagulation reversal, overall mortality (including 30-day and in-hospital mortality), and thromboembolic events. Secondary outcomes were length of hospital and intensive care unit stay and hematoma volume expansion. Data were pooled using a random-effects model. We included 16 eligible studies with a total of 2,977 patients. A statistically significant improvement in hemostatic efficacy rates was in favor of the AA group (risk ratio [RR] 1.10, 95% confidence interval [CI] 1.01-1.20, P = 0.02). Lower overall mortality rates were found in the AA group (RR 0.67, 95% CI 0.51-0.88, P = 0.004). However, no difference was found in 30-day mortality rates (RR 0.82, 95% CI 0.58-1.16, P = 0.26). In terms of thromboembolic events, more events were found in the AA group (RR 1.47, 95% CI 1.01-2.15, P = 0.046). AA was associated with a longer duration of hospital stay compared to 4F-PCC (mean difference [MD] 0.64, 95% CI 0.07-1.22, P = 0.03). Neither a significant difference in length of intensive care unit stay (MD 0.25, 95% CI - 0.36 to 0.86, P = 0.41) nor a significant difference in hematoma volume expansion was reported (MD - 0.89, 95% CI - 3.11 to 1.34, P = 0.435). Our results suggest that AA is superior to 4F-PCC in enhancing the hemostatic efficacy and reducing the overall and in-hospital mortality rates. More thromboembolic events are thought to be associated with the use of AA. However, more studies are required to validate whether the better results of AA in improving hemostatic efficacy are enough to make up for their higher cost and their possible risk of thromboembolic events.
Keywords: Andexanet alfa; Anticoagulants; Intracerebral hemorrhage; Prothrombin complex concentrates; Reversal agents.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors have declared that they do not have any competing interests.
Figures


References
-
- Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124(15):2450–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

