Capivasertib and fulvestrant for patients with HR-positive/HER2-negative advanced breast cancer: analysis of the subgroup of patients from Japan in the phase 3 CAPItello-291 trial
- PMID: 39379782
- PMCID: PMC11717841
- DOI: 10.1007/s12282-024-01640-z
Capivasertib and fulvestrant for patients with HR-positive/HER2-negative advanced breast cancer: analysis of the subgroup of patients from Japan in the phase 3 CAPItello-291 trial
Abstract
Background: In CAPItello-291, capivasertib-fulvestrant significantly improved progression-free survival (PFS) versus placebo-fulvestrant in the overall and PIK3CA/AKT1/PTEN-altered population with hormone receptor-positive (HR-positive)/human epidermal growth factor receptor 2-negative (HER2-negative) advanced breast cancer. Capivasertib-fulvestrant is approved in Japan for the treatment of patients with one or more tumor biomarker alterations (PIK3CA, AKT1 or PTEN). Here, we report outcomes in the CAPItello-291 subgroup of patients from Japan.
Methods: Adults with HR-positive/HER2-negative advanced breast cancer whose disease had relapsed or progressed during or after treatment with an aromatase inhibitor, with or without previous cyclin-dependent kinase 4/6 (CDK4/6) inhibitor therapy, were randomly assigned (1:1 ratio) to receive capivasertib or placebo, plus fulvestrant. The dual primary endpoint was investigator-assessed PFS in the overall and PIK3CA/AKT1/PTEN-altered population. Safety was a secondary endpoint.
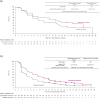
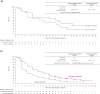
Results: Of 708 patients randomized in CAPItello-291, 78 were from Japan (37 randomized to capivasertib-fulvestrant and 41 to placebo-fulvestrant). In the Japan subgroup, PFS numerically favored the capivasertib-fulvestrant arm (hazard ratio 0.73; 95% CI 0.40-1.28), consistent with the analysis of PFS in the global population. Similarly, in the Japan subgroup of patients with PIK3CA/AKT1/PTEN-altered tumors, PFS favored the capivasertib-fulvestrant arm (hazard ratio 0.65; 95% CI 0.29-1.39), consistent with the global population. The adverse event profile of capivasertib-fulvestrant in the Japan subgroup was broadly similar to that in the global population; no new safety concerns were identified.
Conclusion: Outcomes in the Japan subgroup were broadly similar to those of the global population, supporting the clinical benefit of capivasertib-fulvestrant in treating HR-positive/HER2-negative advanced breast cancer that has progressed on, or after, an endocrine-based regimen.
Keywords: AKT inhibitor; Capivasertib; Hormone receptor-positive breast cancer; Post-CDK4/6.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Eriko Tokunaga has received payment or honoraria from AstraZeneca, Chugai, Daiichi-Sankyo and Eli Lilly. Hiroji Iwata has received consultancy fees and payment or honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, Lilly, MSD and Pfizer; has received consultancy fees from Giliead and has received payment or honoraria from Kyowa Kirin and Taiho. His institution has also received grants from AstraZeneca, Chugai, and Daiichi-Sankyo. Tetsuhiko Taira has received payment or honoraria from Chugai, Daiichi-Sankyo, Eli Lilly, Kyowa Kirin, MSD, and Taiho. Tatsuya Toyama has received lecture fees from Chugai, Daiichi-Sankyo, Pfizer, MSD, Lilly, Novartis, and Taiho. Toshiro Mizuno has received payment or honoraria from AstraZeneca, Ono Pharmaceutical, Daiichi-Sankyo, Pfizer, Lilly, MSD, Eisai, Chugai Pharmaceutical, Kyowa Kirin, and Nippon Kayaku. Akihiko Osaki has received grants from AstraZeneca plc, Chugai Pharmaceutical Co., Ltd, Daiichi-Sankyo co., Ltd., Eisai Co., Ltd., Eli Lilly Japan KK, LabCorp Japan, G.K., MSD K.K., Nippon Kayaku Co., Ltd., Nipro Corp, Novartis Pharma K.K., Sanofi S.A., Taiho Pharmaceutical Co., Ltd.., Takeda Pharmaceutical Co., Ltd and WJOG. They have also received lecture fees from Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo co., Ltd., Eisai Co., Ltd., Eli Lilly Japan KK, Kyowa Kirin Co., Ltd., Nippon Kayaku Co., Ltd. and Pfizer Japan Inc. Seigo Nakamura has received payment or honoraria from AstraZeneca. Rikiya Nakamura has received payment or honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, Eisai and Eli Lilly. Sacha J Howell has received grants from Eli Lily through a joint working initiative, has received consulting fees from AstraZeneca, Eli Lilly and Pfizer, has received payment or honoraria from AstraZeneca, Eli Lilly, Novartis, and Pfizer, and has received support for travel from Novartis. Masakazu Toi has received research grants from Chugai, Takeda, Pfizer, Taiho, JBCRG assoc., KBCRN assoc., Eisai, Eli Lilly and companies, Daiichi-Sankyo, AstraZeneca, Astellas, Shimadzu, Yakult, Nippon Kayaku, AFI technology, Luxonus, Shionogi, GL Science and Sanwa Shurui. They have received fees for lecturing from Chugai, Takeda, Pfizer, Kyowa Kirin, Taiho, Eisai, Daiichi-Sankyo, AstraZeneca, Eli Lilly and companies, MSD, Exact Science, Novartis, Shimadzu, Yakult, Nippon Kayaku, Devicore Medical Japan, Sysmex and advisory board fees from Daiichi-Sankyo, Eli Lilly and companies, BMS, Bertis, Terumo, Kansai Medical Net. Toshiaki Ozaki, Tomoko Sambe, and Gaia Schiavon are employees of AstraZeneca. Mitsuya Itoh and Yasuhiro Yanagita report no conflicts of interest. Research involving human participants and/or animals: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, as well as Good Clinical Practice guidelines and the AstraZeneca policy on bioethics. Informed consent: Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. https://gco.iarc.fr/today/en. Accessed 14 May 2024
-
- Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–37. - PubMed
-
- Toyoda Y, Tabuchi T, Nakayama T, Hojo S, Yoshioka S, Maeura Y. Past trends and future estimation of annual breast cancer incidence in Osaka, Japan. Asian Pac J Cancer Prev. 2016;17(6):2847–52. - PubMed
-
- Gennari A, André F, Barrios C, Cortés J, De Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

