Brain volume loss in relapsing multiple sclerosis: indirect treatment comparisons of available disease-modifying therapies
- PMID: 39379875
- PMCID: PMC11460132
- DOI: 10.1186/s12883-024-03888-6
Brain volume loss in relapsing multiple sclerosis: indirect treatment comparisons of available disease-modifying therapies
Abstract
Background: Brain volume loss (BVL) has been identified as a predictor of disability progression in relapsing multiple sclerosis (RMS). As many available disease-modifying treatments (DMTs) have shown an effect on slowing BVL, this is becoming an emerging clinical endpoint in RMS clinical trials.
Methods: In this study, a systematic literature review was conducted to identify BVL results from randomized controlled trials of DMTs in RMS. Indirect treatment comparisons (ITCs) were conducted to estimate the relative efficacy of DMTs on BVL using two approaches: a model-based meta-analysis (MBMA) with adjustment for measurement timepoint and DMT dosage, and a network meta-analysis (NMA).
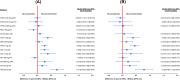
Results: In the MBMA, DMTs associated with significantly reduced BVL versus placebo at two years included fingolimod (mean difference [MD] = 0.25; 95% confidence interval [CI] = 0.15 - 0.36), ozanimod (MD = 0.26; 95% CI = 0.12 - 0.41), teriflunomide (MD = 0.38; 95% CI = 0.20 - 0.55), alemtuzumab (MD = 0.38; 95% CI = 0.10 - 0.67) and ponesimod (MD = 0.71; 95% CI = 0.48 - 0.95), whereas interferons and natalizumab performed the most poorly. The results of NMA analysis were generally comparable with those of the MBMA.
Conclusions: Limitations of these analyses included the potential for confounding due to pseudoatrophy, and a lack of long-term clinical data for BVL. Our findings suggest that important differences in BVL may exist between DMTs. Continued investigation of BVL in studies of RMS is important to complement traditional disability endpoints, and to foster a better understanding of the mechanisms by which DMTs can slow BVL.
Keywords: Brain volume loss; Disease modifying therapy; Magnetic resonance imaging; Model-based meta-analysis; Multiple sclerosis; Network meta-analysis; Systematic literature review.
© 2024. The Author(s).
Conflict of interest statement
ESH, VV, AOR and SS are employees of EVERSANA, and MLZ is an employee of Certara USA. AK, HL, KG and MAT are employees of Janssen, part of Johnson and Johnson group of companies and may hold stock/stock options of Johnson and Johnson. BH has previously received honoraria from EVERSANA for methodologic guidance related to the conduct of systematic reviews and meta-analysis. RZ has received personal compensation from Bristol Myers Squibb, EMD Serono, Sanofi, Keystone Heart, Janssen, 415 Capital, Mapi Pharma, Protembis and Novartis for speaking and consultant fees. He received financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Octave, Mapi Pharma, Keystone Heart, Protembis and V-WAVE Medical. The preparation of this manuscript was funded by Janssen.
Figures

References
-
- De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–76. - PubMed
-
- Sormani MP, Arnold D, De Stefano N. Combined MRI measure of active lesions and brain atrophy as a surrogate for disability in multiple sclerosis: a meta-analysis of randomized trials (P07.096). Neurology. 2016;80(7 Supplement):P07.096.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

