SMYD1 modulates the proliferation of multipotent cardiac progenitor cells derived from human pluripotent stem cells during myocardial differentiation through GSK3β/β-catenin&ERK signaling
- PMID: 39380045
- PMCID: PMC11462858
- DOI: 10.1186/s13287-024-03899-7
SMYD1 modulates the proliferation of multipotent cardiac progenitor cells derived from human pluripotent stem cells during myocardial differentiation through GSK3β/β-catenin&ERK signaling
Abstract
Background: The histone-lysine N-methyltransferase SMYD1, which is specific to striated muscle, plays a crucial role in regulating early heart development. Its deficiency has been linked to the occurrence of congenital heart disease. Nevertheless, the precise mechanism by which SMYD1 deficiency contributes to congenital heart disease remains unclear.
Methods: We established a SMYD1 knockout pluripotent stem cell line and a doxycycline-inducible SMYD1 expression pluripotent stem cell line to investigate the functions of SMYD1 utilizing an in vitro-directed myocardial differentiation model.
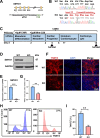
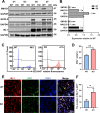
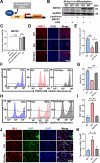
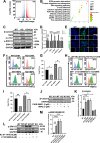
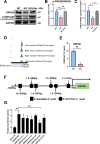
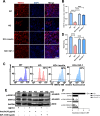
Results: Cardiomyocytes lacking SMYD1 displayed drastically diminished differentiation efficiency, concomitant with heightened proliferation capacity of cardiac progenitor cells during the early cardiac differentiation stage. These cellular phenotypes were confirmed through experiments inducing the re-expression of SMYD1. Transcriptome sequencing and small molecule inhibitor intervention suggested that the GSK3β/β-catenin&ERK signaling pathway was involved in the proliferation of cardiac progenitor cells. Chromatin immunoprecipitation demonstrated that SMYD1 acted as a transcriptional activator of GSK3β through histone H3 lysine 4 trimethylation. Additionally, dual-luciferase analyses indicated that SMYD1 could interact with the promoter region of GSK3β, thereby augmenting its transcriptional activity. Moreover, administering insulin and Insulin-like growth factor 1 can enhance the efficacy of myocardial differentiation in SMYD1 knockout cells.
Conclusions: Our research indicated that the participation of SMYD1 in the GSK3β/β-catenin&ERK signaling cascade modulated the proliferation of cardiac progenitor cells during myocardial differentiation. This process was partly reliant on the transcription of GSK3β. Our research provided a novel insight into the genetic modification effect of SMYD1 during early myocardial differentiation. The findings were essential to the molecular mechanism and potential interventions for congenital heart disease.
Keywords: GSK3β; Histone modification; Human pluripotent stem cells; Myocardial differentiation; SMYD1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
Similar articles
-
CHD4 and SMYD1 repress common transcriptional programs in the developing heart.Development. 2024 Apr 15;151(8):dev202505. doi: 10.1242/dev.202505. Epub 2024 May 3. Development. 2024. PMID: 38619323 Free PMC article.
-
Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7871-E7880. doi: 10.1073/pnas.1800680115. Epub 2018 Jul 30. Proc Natl Acad Sci U S A. 2018. PMID: 30061404 Free PMC article.
-
Modulation of chromatin remodeling proteins SMYD1 and SMARCD1 promotes contractile function of human pluripotent stem cell-derived ventricular cardiomyocyte in 3D-engineered cardiac tissues.Sci Rep. 2019 May 16;9(1):7502. doi: 10.1038/s41598-019-42953-w. Sci Rep. 2019. PMID: 31097748 Free PMC article.
-
Unraveling the Inconsistencies of Cardiac Differentiation Efficiency Induced by the GSK3β Inhibitor CHIR99021 in Human Pluripotent Stem Cells.Stem Cell Reports. 2018 Jun 5;10(6):1851-1866. doi: 10.1016/j.stemcr.2018.03.023. Epub 2018 Apr 26. Stem Cell Reports. 2018. PMID: 29706502 Free PMC article.
-
Crystal structure of cardiac-specific histone methyltransferase SmyD1 reveals unusual active site architecture.J Biol Chem. 2010 Dec 24;285(52):40635-44. doi: 10.1074/jbc.M110.168187. Epub 2010 Oct 12. J Biol Chem. 2010. PMID: 20943667 Free PMC article.
Cited by
-
Heart Morphogenesis Requires Smyd1b for Proper Incorporation of the Second Heart Field in Zebrafish.Genes (Basel). 2025 Jan 4;16(1):52. doi: 10.3390/genes16010052. Genes (Basel). 2025. PMID: 39858599 Free PMC article.
References
-
- Benjamin E, Muntner P, Alonso A, Bittencourt M, Callaway C, Carson A, et al. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019;139:e56–528. - PubMed
-
- Wang K, Li Y, Qiang T, Chen J, Wang X. Role of epigenetic regulation in myocardial ischemia/reperfusion injury. Pharmacol Res. 2021;170: 105743. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

