DEMINING: A deep learning model embedded framework to distinguish RNA editing from DNA mutations in RNA sequencing data
- PMID: 39380061
- PMCID: PMC11463134
- DOI: 10.1186/s13059-024-03397-2
DEMINING: A deep learning model embedded framework to distinguish RNA editing from DNA mutations in RNA sequencing data
Abstract
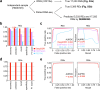
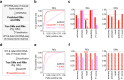
Precise calling of promiscuous adenosine-to-inosine RNA editing sites from transcriptomic datasets is hindered by DNA mutations and sequencing/mapping errors. Here, we present a stepwise computational framework, called DEMINING, to distinguish RNA editing and DNA mutations directly from RNA sequencing datasets, with an embedded deep learning model named DeepDDR. After transfer learning, DEMINING can also classify RNA editing sites and DNA mutations from non-primate sequencing samples. When applied in samples from acute myeloid leukemia patients, DEMINING uncovers previously underappreciated DNA mutation and RNA editing sites; some associated with the upregulated expression of host genes or the production of neoantigens.
Keywords: AML; DNA mutation; Deep learning; IDR; Neoantigens; RNA editing; RNA-seq; Transfer learning.
© 2024. The Author(s).
Conflict of interest statement
F.N. and L.Y. have filed a patent application (202310642373.8) relating to this work through Children’s Hospital of Fudan University. However, the patent does not restrict the educational, research, and not-for-profit purposes. The remaining authors declare no competing interests.
Figures



Similar articles
-
Nanopore sequencing to detect A-to-I editing sites.Methods Enzymol. 2025;710:187-205. doi: 10.1016/bs.mie.2024.11.028. Epub 2024 Dec 4. Methods Enzymol. 2025. PMID: 39870444
-
Biochemical and Transcriptome-Wide Identification of A-to-I RNA Editing Sites by ICE-Seq.Methods Enzymol. 2015;560:331-53. doi: 10.1016/bs.mie.2015.03.014. Epub 2015 Jul 9. Methods Enzymol. 2015. PMID: 26253977
-
RES-Scanner: a software package for genome-wide identification of RNA-editing sites.Gigascience. 2016 Aug 18;5(1):37. doi: 10.1186/s13742-016-0143-4. Gigascience. 2016. PMID: 27538485 Free PMC article.
-
Computational approaches for detection and quantification of A-to-I RNA-editing.Methods. 2019 Mar 1;156:25-31. doi: 10.1016/j.ymeth.2018.11.011. Epub 2018 Nov 20. Methods. 2019. PMID: 30465820 Review.
-
Using mouse models to unlock the secrets of non-synonymous RNA editing.Methods. 2019 Mar 1;156:40-45. doi: 10.1016/j.ymeth.2018.10.016. Epub 2018 Oct 26. Methods. 2019. PMID: 30827465 Free PMC article. Review.
Cited by
-
In silico and experimental approaches for validating RNA editing events in transcriptomes.RNA Biol. 2024 Jan;21(1):31-36. doi: 10.1080/15476286.2024.2432729. Epub 2024 Nov 24. RNA Biol. 2024. PMID: 39582096 Free PMC article.
-
Specific and efficient RNA A-to-I editing through cleavage of an ADAR inhibitor.Nat Biotechnol. 2025 Mar 26. doi: 10.1038/s41587-025-02591-2. Online ahead of print. Nat Biotechnol. 2025. PMID: 40140558
-
A novel sequence-based transformer model architecture for integrating multi-omics data in preterm birth risk prediction.NPJ Digit Med. 2025 Aug 20;8(1):536. doi: 10.1038/s41746-025-01942-2. NPJ Digit Med. 2025. PMID: 40835718 Free PMC article.
References
-
- Chen LL, Yang L. ALUternative regulation for gene expression. Trends Cell Biol. 2017;27:480–90. - PubMed
MeSH terms
Grants and funding
- 31925011/National Natural Science Foundation of China
- 2019YFA0802804, 2021YFA1300503/the Ministry of Science and Technology of China
- 23JS1400300, 23DX1900102/Shanghai Municipal Science and Technology Commission
- XDB38040300/Chinese Academy of Sciences
- 23YF1407400/Shanghai Municipal Science and Technology Commission
LinkOut - more resources
Full Text Sources

