IL-7Rα on CD4+ T cells is required for their survival and the pathogenesis of experimental autoimmune encephalomyelitis
- PMID: 39380064
- PMCID: PMC11460225
- DOI: 10.1186/s12974-024-03224-2
IL-7Rα on CD4+ T cells is required for their survival and the pathogenesis of experimental autoimmune encephalomyelitis
Abstract
Background: The IL-7 receptor alpha (IL-7Rα) binds both IL-7 and thymic stromal lymphopoietin (TSLP). IL-7Rα is essential for the development and survival of naive CD4+ T cells and their differentiation to effector/memory CD4+ T cells. Mice lacking IL-7Rα have severe lymphopenia and are resistant to experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis. However, it has been reported that IL-7Rα on peripheral CD4+ T cells is disposable for their maintenance and EAE pathogenesis, which does not align with the body of knowledge on the role of IL-7Rα in the biology of CD4+ T cells. Given that a definitive study on this important topic is lacking, we revisited it using a novel approach, an inducible knockout of the IL-7Rα gene in CD4+ T cells.
Methods: We generated Il7rafl/fl/CD4CreERT2 double transgenic mouse line (henceforth CD4ΔIl7ra), susceptible to tamoxifen-induced knockout of the IL-7Rα gene in CD4+ T cells. CD4ΔIl7ra mice were immunized with MOG35 - 55 for EAE induction and monitored for disease development. The expression of IL-7Rα, CD4+ T cell numbers, and MOG35 - 55-specific CD4+ T cell response was evaluated in the central nervous system (CNS) and lymphoid tissues by flow cytometry. Additionally, splenocytes of CD4ΔIl7ra mice were stimulated with MOG35 - 55 to assess their proliferative response and cytokine production by T helper cells.
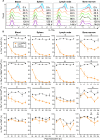
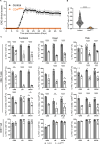
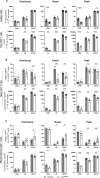
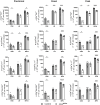
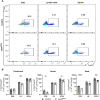
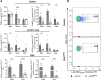
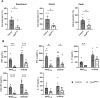
Results: Loss of IL-7Rα from the surface of CD4+ T cells in CD4ΔIl7ra mice was virtually complete several days after tamoxifen treatment. The loss of IL-7Rα in CD4+ T cells led to a gradual and substantial decrease in their numbers in both non-immunized and immunized CD4ΔIl7ra mice, followed by slow repopulation up to the initial numbers. CD4ΔIl7ra mice did not develop EAE. We found a decrease in the total numbers of TNF-, IFN-γ-, IL-17 A-, and GM-CSF-producing CD4+ T cells and regulatory T cells in the spleens and CNS of immunized CD4ΔIl7ra mice. Tracking MOG35 - 55-specific CD4+ T cells revealed a significant reduction in their numbers in CD4ΔIl7ra mice and decreased proliferation and cytokine production in response to MOG35 - 55.
Conclusion: Our study demonstrates that IL-7Rα on peripheral CD4+ T cells is essential for their maintenance, immune response, and EAE pathogenesis.
Keywords: CD4+ T cell; Experimental autoimmune encephalomyelitis; IL-7 receptor alpha; Multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Regulation of effector function of CNS autoreactive CD4 T cells through inhibitory receptors and IL-7Rα.J Neuroinflammation. 2016 Dec 3;13(1):302. doi: 10.1186/s12974-016-0768-3. J Neuroinflammation. 2016. PMID: 27912762 Free PMC article.
-
IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis.J Immunol. 2015 Sep 1;195(5):1974-83. doi: 10.4049/jimmunol.1403135. Epub 2015 Jul 29. J Immunol. 2015. PMID: 26223651 Free PMC article.
-
Characterization of myelin oligodendrocyte glycoprotein (MOG)35-55-specific CD8+ T cells in experimental autoimmune encephalomyelitis.Chin Med J (Engl). 2019 Dec 20;132(24):2934-2940. doi: 10.1097/CM9.0000000000000551. Chin Med J (Engl). 2019. PMID: 31855963 Free PMC article.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Control of experimental autoimmune encephalomyelitis by CD4+ suppressor T cells: peripheral versus in situ immunoregulation.J Neuroimmunol. 2007 Nov;191(1-2):61-9. doi: 10.1016/j.jneuroim.2007.09.010. Epub 2007 Sep 27. J Neuroimmunol. 2007. PMID: 17900707 Free PMC article. Review.
Cited by
-
Construction of epilepsy diagnosis model based on cell senescence-related genes and its potential mechanism.Front Neurol. 2025 May 30;16:1555586. doi: 10.3389/fneur.2025.1555586. eCollection 2025. Front Neurol. 2025. PMID: 40520613 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

