Elevated plasma and CSF neurofilament light chain concentrations are stabilized in response to mutant huntingtin lowering in the brains of Huntington's disease mice
- PMID: 39380076
- PMCID: PMC11460072
- DOI: 10.1186/s40035-024-00443-8
Elevated plasma and CSF neurofilament light chain concentrations are stabilized in response to mutant huntingtin lowering in the brains of Huntington's disease mice
Abstract
Background: Therapeutic approaches aimed at lowering toxic mutant huntingtin (mHTT) levels in the brain can reverse disease phenotypes in animal models of Huntington's disease (HD) and are currently being evaluated in clinical trials. Sensitive and dynamic response biomarkers are needed to assess the efficacy of such candidate therapies. Neurofilament light chain (NfL) is a biomarker of neurodegeneration that increases in cerebrospinal fluid (CSF) and blood with progression of HD. However, it remains unknown whether NfL in biofluids could serve as a response biomarker for assessing the efficacy of disease-modifying therapies for HD.
Methods: Longitudinal plasma and cross-sectional CSF samples were collected from the YAC128 transgenic mouse model of HD and wild-type (WT) littermate control mice throughout the natural history of disease. Additionally, biofluids were collected from YAC128 mice following intracerebroventricular administration of an antisense oligonucleotide (ASO) targeting the mutant HTT transgene (HTT ASO), at ages both before and after the onset of disease phenotypes. NfL concentrations in plasma and CSF were quantified using ultrasensitive single-molecule array technology.
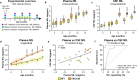
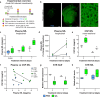
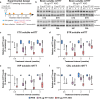
Results: Plasma and CSF NfL concentrations were significantly elevated in YAC128 compared to WT littermate control mice from 9 months of age. Treatment of YAC128 mice with either 15 or 50 µg HTT ASO resulted in a dose-dependent, allele-selective reduction of mHTT throughout the brain at a 3-month interval, which was sustained with high-dose HTT ASO treatment for up to 6 months. Lowering of brain mHTT prior to the onset of regional brain atrophy and HD-like motor deficits in this model had minimal effect on plasma NfL at either dose, but led to a dose-dependent reduction of CSF NfL. In contrast, initiating mHTT lowering in the brain after the onset of neuropathological and behavioural phenotypes in YAC128 mice resulted in a dose-dependent stabilization of NfL increases in both plasma and CSF.
Conclusions: Our data provide evidence that the response of NfL in biofluids is influenced by the magnitude of mHTT lowering in the brain and the timing of intervention, suggesting that NfL may serve as a promising exploratory response biomarker for HD.
Keywords: Antisense oligonucleotide; Biofluids; Cerebrospinal fluid; Huntingtin lowering; Huntington's disease; Neurofilament light chain; Plasma; Response biomarker.
© 2024. The Author(s).
Conflict of interest statement
DJH and PM are paid employees of F. Hoffmann-La Roche. EJW reports grants from CHDI Foundation and European Huntington’s Disease Network. EJW reports consultancy / advisory board memberships with Alnylam, Annexon, Remix Therapeutics, F. Hoffman-La Roche Ltd, Ionis Pharmaceuticals, PTC Therapeutics, Skyhawk Therapeutics, Takeda, Teitur Trophics, Triplet Therapeutics, Uniqure, Wave Life Sciences, and Vico Therapeutics. He is an investigator on the AMT-130 program. All honoraria for these consultancies were paid through the offices of UCL Consultants Ltd., a wholly owned subsidiary of University College London. BRL is a founder and CEO of Incisive Genetics Inc. MRH is the CEO of Prilenia Therapeutics and serves on the public boards of Ionis Pharmaceuticals, Oxford Biomedica, AbCellera and 89bio.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

