Monocyte subsets in breast cancer patients under treatment with aromatase inhibitor and mucin-1 cancer vaccine
- PMID: 39380101
- PMCID: PMC11460172
- DOI: 10.1186/s12967-024-05659-w
Monocyte subsets in breast cancer patients under treatment with aromatase inhibitor and mucin-1 cancer vaccine
Abstract
Background: Monocytes comprise subsets of classical, intermediate and non-classical monocytes with distinct anti- or pro-tumor effects in breast cancer (BC). They are modulated by estrogen, and can contribute to BC control by endocrine therapy in preclinical models.
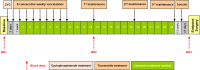
Methods: To elucidate whether changes in monocyte subsets are associated with treatment and response, we investigated peripheral blood samples of 73 postmenopausal women with estrogen receptor (ER) positive BC, who received aromatase inhibitor therapy with or without the mucin-1 vaccine tecemotide in the ABCSG34 trial. Blood was retrieved at baseline, midterm and end of therapy, and was analyzed for the distribution and ER expression of monocyte subsets by flow cytometry.
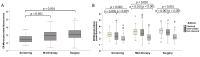
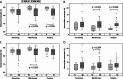
Results: When 40 healthy, age-matched women were compared with BC patients before treatment start, ER levels of monocytes did not differ, yet patients presented with a higher frequency of classical and fewer non-classical monocytes. Endocrine therapy triggered a significant increase in ER levels in all monocyte subsets, without affecting subset distribution. Vaccination had no overall impact on subset frequency and ER expression. Yet, a shift from intermediate to classical monocytes during therapy correlated with changes in plasma cytokines and chemokines and was significantly associated with low residual cancer burden in vaccinated patients. Without tecemotide, baseline ER levels in classical monocytes were significantly higher in women with good response to endocrine therapy.
Conclusions: This study identified classical monocytes to be associated with ER positive BC and with patient response to neoadjuvant endocrine treatment and cancer vaccination.
Keywords: Aromatase inhibitor; Breast cancer; Estrogen receptor; Letrozole; Monocyte; Stimuvax; Tecemotide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Demicheli R, Ardoino I, Boracchi P, Coradini D, Agresti R, Ferraris C, Gennaro M, Hrushesky WJ, Biganzoli E. Recurrence and mortality according to estrogen receptor status for breast cancer patients undergoing conservative surgery. Ipsilateral breast tumour recurrence dynamics provides clues for tumour biology within the residual breast. BMC Cancer. 2010;10:656. - PMC - PubMed
-
- Burstein HJ. Systemic therapy for Estrogen Receptor-Positive, HER2-Negative breast Cancer. N Engl J Med. 2020;383:2557–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

