Prodrug Nanomedicine for Synovium Targeted Therapy of Inflammatory Arthritis: Insights from Animal Model and Human Synovial Joint Fluid
- PMID: 39380387
- PMCID: PMC11616258
- DOI: 10.1002/adhm.202401936
Prodrug Nanomedicine for Synovium Targeted Therapy of Inflammatory Arthritis: Insights from Animal Model and Human Synovial Joint Fluid
Abstract
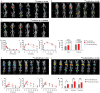
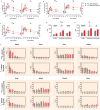
Many patients cannot tolerate low-dose weekly methotrexate (MTX) therapy for inflammatory arthritis treatment due to life-threatening toxicity. Although biologics offer a target-specific therapy, it raises the risk of serious infections and even cancer due to immune system suppression. We introduce an anti-inflammatory arthritis MTX ester prodrug using a long-circulating biocompatible polymeric macromolecule: folic acid (FA) functionalized hyperbranched polyglycerol (HPG). In vitro the drug MTX is incrementally released through pH and enzymatic degradation over 2 weeks. The role of matrix metalloproteinases (MMPs) in site-specific prodrug activation was verified using synovial fluid (SF) of 26 rheumatology patients and 4 healthy controls. Elevated levels of specific MMPs-markers of joint inflammation-positively correlated with enhanced prodrug release explained by acid-catalyzed hydrolysis of esters by proteases. Intravenously administered 111In-radiolabeled prodrug confirmed by SPECT/CT imaging that it accumulated preferentially in inflamed joints while reducing off-target side-effects in a mouse model of rheumatoid arthritis (RA). Added FA as a targeting vector prolonged prodrug action; prodrug with 4x less MTX applied every 2 weeks was as effective as weekly MTX therapy. The preclinical results suggest a prodrug-based strategy for the treatment of inflammatory joint diseases, with potential for other chronic inflammatory diseases and cancer.
Keywords: drug delivery system; methotrexate; polymer; prodrug; synovial fluid.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Clunie G., Wilkinson N., Nikiphorou E., Jadon D., Oxford Handbook of Rheumatology, Oxford University Press, Oxford: 2018.
-
- Scott D. L., Wolfe F., Huizinga T. W., Lancet 2010, 376, 1094. - PubMed
-
- d'Arcy R., Tirelli N., Polym. Adv. Technol. 2014, 25, 478.
-
- Andersson S. E., Lexmüller K., Johansson A., Ekström G. M., J. Rheumatol. 1999, 26, 2018. - PubMed
-
- van der Heijden J. W., Oerlemans R., Dijkmans B. A., Qi H., van der Laken C. J., Lems W. F., Jackman A. L., Kraan M. C., Tak P. P., Ratnam M., Jansen G., Arthritis Rheum. 2009, 60, 12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

