Economic and societal burden of myasthenia gravis in Denmark, Finland, and Sweden: A population-based registry study
- PMID: 39380430
- PMCID: PMC11555013
- DOI: 10.1111/ene.16511
Economic and societal burden of myasthenia gravis in Denmark, Finland, and Sweden: A population-based registry study
Abstract
Background and purpose: Health care resource utilization (HCRU) and the economic burden of myasthenia gravis (MG) are significant, but existing studies rarely include comprehensive nationwide data. We examined HCRU and direct and indirect costs associated with MG overall and by disease severity in Denmark, Finland, and Sweden.
Methods: Data were collected retrospectively from nationwide health and social care registries. All individuals ≥18 years of age with ≥2 International Classification of Diseases diagnoses of MG between 2000 and 2020 were included. HCRU, direct (inpatient and outpatient contacts, medication) and indirect costs (early retirement, sick leave, death), and associated factors were calculated.
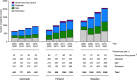
Results: The full study cohort comprised 8622 people with MG (pwMG). Mean annual numbers of all-cause secondary health care contacts for pwMG were 3.4 (SD = 8.3), 7.0 (SD = 12.3), and 2.9 (SD = 3.9), with mean annual total costs of €12,185, €9036, and €5997 per person in Denmark, Finland, and Sweden, respectively. Inpatient periods, involving 77%-89% of study participants in the three countries, contributed most to direct costs, whereas the majority of indirect costs resulted from early retirement in Denmark and Finland, and sick leave periods in Sweden. Mean annual total costs were highest with very severe MG (€19,570-€33,495 per person across the three countries). Female sex and comorbidities, such as mental and behavioral disorders and severe infections, were also associated with higher total costs.
Conclusions: This population-based study shows a high level of HCRU and a significant direct and indirect economic burden of MG across three Nordic countries, especially for severe forms of MG.
Keywords: direct costs; economic burden; health care resource utilization; indirect costs; myasthenia gravis.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
F.P. has received funds from Janssen, Merck KGaA, UCB, Chugai, Lundbeck, Roche, and Novartis. J.V. has received funds from Roche, Sanofi Genzyme, Sarepta Therapeutics, Novartis Pharma, Fulcrum Therapeutics, Biogen, Lupin, Amicus, Regeneron, Argenx, UCB Biopharma, ML Biopharma, Atamyo, Horizon Therapeutics, Dyne Therapeutics Research, Alexion Pharmaceuticals, Edgewise Therapeutics, Genethon, and Janssen Pharmaceutical. J.M. is an employee of MedEngine. F.B. is an employee and stockholder of UCB Pharma, Copenhagen, Denmark. I.L.‐S. is an employee of UCB Pharma, Stockholm, Sweden. D.P. was an employee of UCB Pharma, Brussels, Belgium. E.T. was employed by Significance Consulting and contracted by UCB Pharma, Copenhagen, Denmark, at the time of the study. A.V. is an employee of MedEngine. R.‐M.V. is an employee of MedEngine. T.Y. is an employee and stockholder of MedEngine and MedEngine DK. S.A. has received funds from Merck, Roche, Biogen, Novartis, UCB Pharma, and Lundbeck.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

