The Immune Microenvironment in Prostate Cancer: A Comprehensive Review
- PMID: 39380471
- PMCID: PMC12140600
- DOI: 10.1159/000541881
The Immune Microenvironment in Prostate Cancer: A Comprehensive Review
Abstract
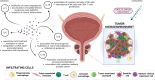
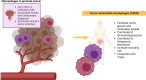
Background: Prostate cancer (PCa) is a malignancy with significant immunosuppressive properties and limited immune activation. This immunosuppression is linked to reduced cytotoxic T cell activity, impaired antigen presentation, and elevated levels of immunosuppressive cytokines and immune checkpoint molecules. Studies demonstrate that cytotoxic CD8+ T cell infiltration correlates with improved survival, while increased regulatory T cells (Tregs) and tumor-associated macrophages (TAMs) are associated with worse outcomes and therapeutic resistance. Th1 cells are beneficial, whereas Th17 cells, producing interleukin-17 (IL-17), contribute to tumor progression. Tumor-associated neutrophils (TANs) and immune checkpoint molecules, such as PD-1/PD-L1 and T cell immunoglobulin-3 (TIM-3) are also linked to advanced stages of PCa. Chemotherapy holds promise in converting the "cold" tumor microenvironment (TME) to a "hot" one by depleting immunosuppressive cells and enhancing tumor immunogenicity.
Summary: This comprehensive review examines the immune microenvironment in PCa, focusing on the intricate interactions between immune and tumor cells in the TME. It highlights how TAMs, Tregs, cytotoxic T cells, and other immune cell types contribute to tumor progression or suppression and how PCa's low immunogenicity complicates immunotherapy.
Key messages: The infiltration of cytotoxic CD8+ T cells and Th1 cells correlates with better outcomes, while elevated T regs and TAMs promote tumor growth, metastasis, and resistance. TANs and natural killer (NK) cells exhibit dual roles, with higher NK cell levels linked to better prognoses. Immune checkpoint molecules like PD-1, PD-L1, and TIM-3 are associated with advanced disease. Chemotherapy can improve tumor immunogenicity by depleting T regs and myeloid-derived suppressor cells, offering therapeutic promise.
Keywords: CD4; CD8; Immunology; Immunotherapy; Metastatic; Myeloid-derived suppressor cells; Neutrophils; Prostate tumor; T cells; Tertiary lymphoid structures; Tumor-associated macrophages.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The Rene Novysedlak, Robin Bartolini, Lily Koumbas Foley, Miray Güney, Majd Al Khouri, Iva Benesova, Andrej Ozaniak, Vojtech Novak, Stepan Vesely, Pavel Pacas, Tomas Buchler, and Zuzana Ozaniak Strizova declare no conflict of interest. Tomas Buchler declares following research support: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Merck KGaA, MSD, and Novartis; consulting fees from Bristol Myers Squibb, Astellas, Janssen, and Sanofi/Aventis; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol-Myers Squibb, AstraZeneca, Roche, Servier, Accord, MSD, and Pfizer. All unrelated to the present paper.
Figures




References
-
- Sun BL. Immunotherapy in treatment of metastatic prostate cancer: an approach to circumvent immunosuppressive tumor microenvironment. Prostate. 2021;81(15):1125–34. - PubMed
-
- Apusiga K. Immune cell infiltration-based prognosis in prostate cancer: a review of current knowledge. Bull Natl Res Cent. 2023;47(1):131.
-
- Dong L, Myers KV, Pienta KJ. Understanding the tumor-immune microenvironment in prostate cancer. Curr Opin Oncol. 2021;33(3):231–7. - PubMed
-
- Liu J, Li Y, Yang D, Yang C, Mao L. Current state of biomarkers for the diagnosis and assessment of treatment efficacy of prostate cancer. Discov Med. 2019;27(150):235–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

